Separating method of 2-chloropyridine and 3-chloropyridine
A separation method, the technology of chloropyridine, applied in chemical instruments and methods, organic chemical methods, preparation of organic compounds, etc., can solve the problems of complex equipment, high operation risk factor, high energy consumption, etc., and achieve simple operation and safe operation Good, energy-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
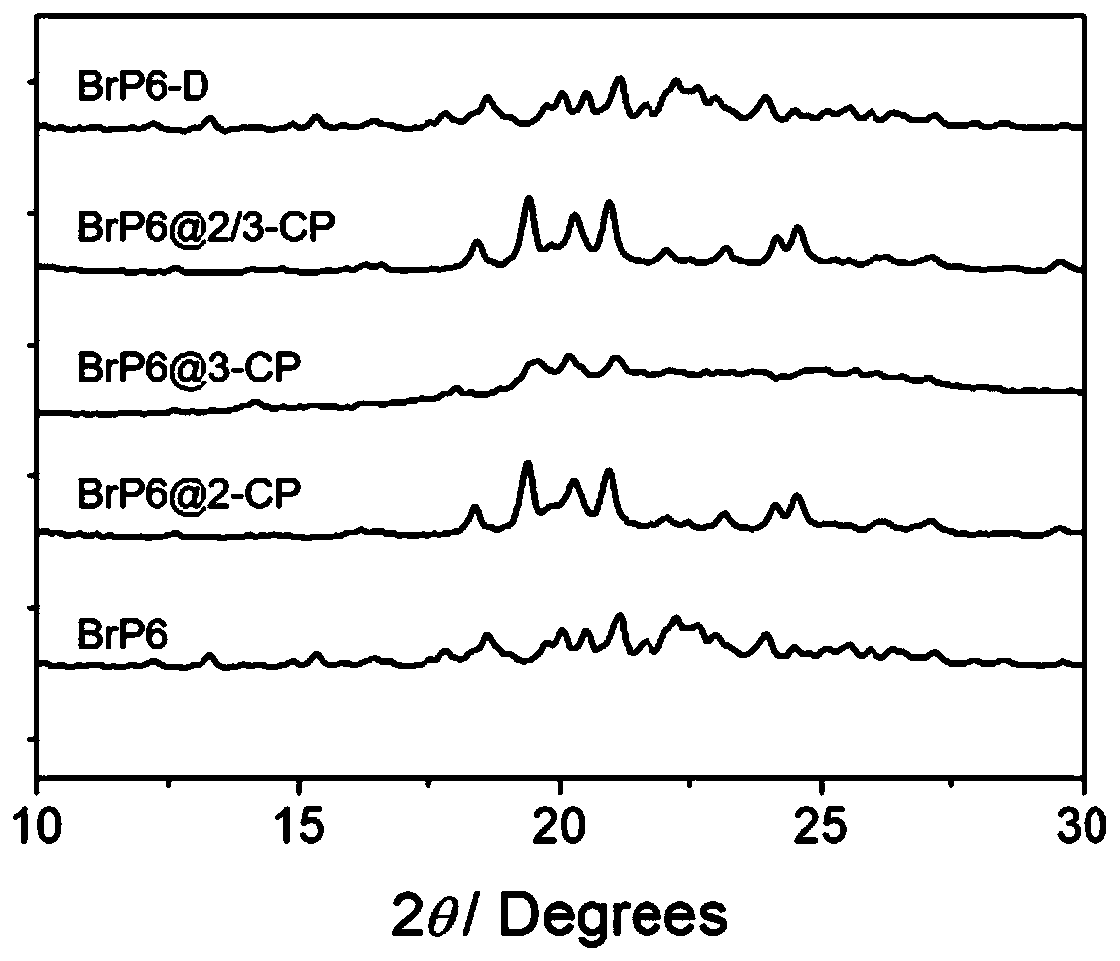
[0029] Preparation of bis-dibromoethoxypillar[6]arene crystal material: Bis-dibromoethoxypillar[6]arene was prepared using 1,1-dibromo-p-phenylenediethyl ether.
[0030] 1,1-Dibromo-p-phenylenediethyl ether (6.74 g, 23.0 mmol) was added to 100 mL of 1,2-dichloroethane, and BF 3 ·O(C 2 h 5 ) 2 (23.0~23.5mmol), the mixture was stirred at 25°C for 20~30 minutes, quenched with saturated sodium bicarbonate solution to end the reaction, washed twice with deionized water, and the organic phase was concentrated under reduced pressure to obtain the crude product, which was used for Purification by flash column chromatography (petroleum ether / dichloromethane volume ratio = 1:2) gave bis-dibromoethoxycolum[6]arene (1.20 g, 16.9% yield) as a white solid. The white solid was placed in a vacuum oven at 120° C. overnight to obtain the activated bis-dibromoethoxypillar[6]arene crystal material as a white powder, denoted as BrP6.
[0031] The product characterization data prepared in this ...
Embodiment 2
[0035] Adsorption of 2-chloropyridine or 3-chloropyridine by bis-dibromoethoxy column[6]arene crystal material: take two 20mL strain bottles, add 2mL 2-chloropyridine and 2mL 3-chloropyridine respectively, and name For BrP6@2-CP and BrP6@3-CP, respectively take 30mg of bis-dibromoethoxy column[6]arene crystal material and place them in two 5mL strain bottles, and place two open 5mL strain bottles in In two 20mL strain bottles, seal the 20mL strain bottles well, place them at room temperature at 25°C for 48 hours, and place the obtained powder in an oven at 40°C for 30 minutes.
[0036] The product characterization data prepared in this embodiment are as follows:
[0037] BrP6@2-CP, 1H NMR (400MHz, CDCl3, 298K, ppm) δ8.42–8.39 (m, 2H), 7.66 (td, J = 7.7, 2.0Hz, 2H), 7.34 (dt, J = 8.1, 0.9Hz, 2H), 7.23(ddd,J=7.4,4.9,1.0Hz,2H),6.78(s,12H),4.16(t,J=5.8Hz,24H),3.87(s,12H),3.55(t,J=5.8Hz ,24H).
[0038] BrP6@3-CP, 1 H NMR (400MHz, CDCl 3 ,298K,ppm)δ8.59(d,J=2.5Hz,2H),8.50(d,J=...
Embodiment 3
[0042] Adsorption of the mixture of 2-chloropyridine and 3-chloropyridine with a volume ratio of 1:1 by bis-dibromoethoxy column[6]arene crystal material: take a 20mL seed bottle, add 1mL 2-chloropyridine and 1mL 3 -Chloropyridine, named BrP6@2 / 3-CP, take 30mg of bisdiethoxy column[6]arene crystal material and place it in a 5mL strain bottle, and put the open 5mL strain bottle into the above 20mL strain bottle In the 20mL strain bottle, seal it well, and place it at room temperature at 25°C for 48 hours, and place the obtained powder in an oven at 40°C for 30 minutes.
[0043] The product characterization data prepared in this embodiment are as follows:
[0044] BrP6@2 / 3-CP, 1 H NMR (400MHz, CDCl 3 ,298K,ppm)δ8.40(dd,J=4.9,1.5Hz,2H),7.66(td,J=7.9,2.0Hz,2H),7.34(d,J=8.0Hz,2H),7.23(ddd ,J=7.4,4.9,0.9Hz,2H),6.78(s,12H),4.16(t,J=5.8Hz,24H),3.87(s,12H),3.55(t,J=5.8Hz,24H) .
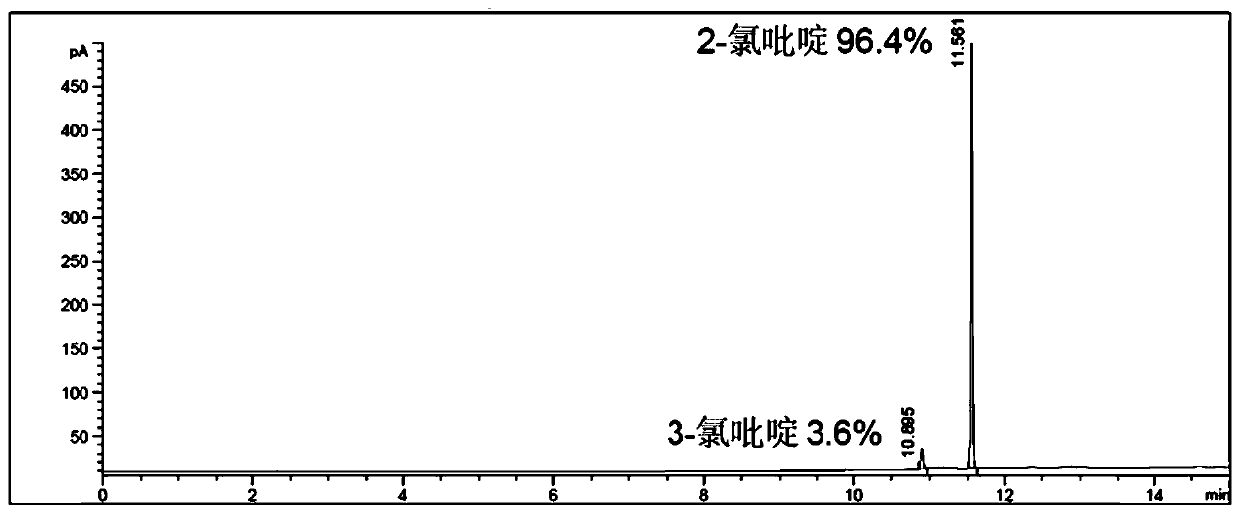
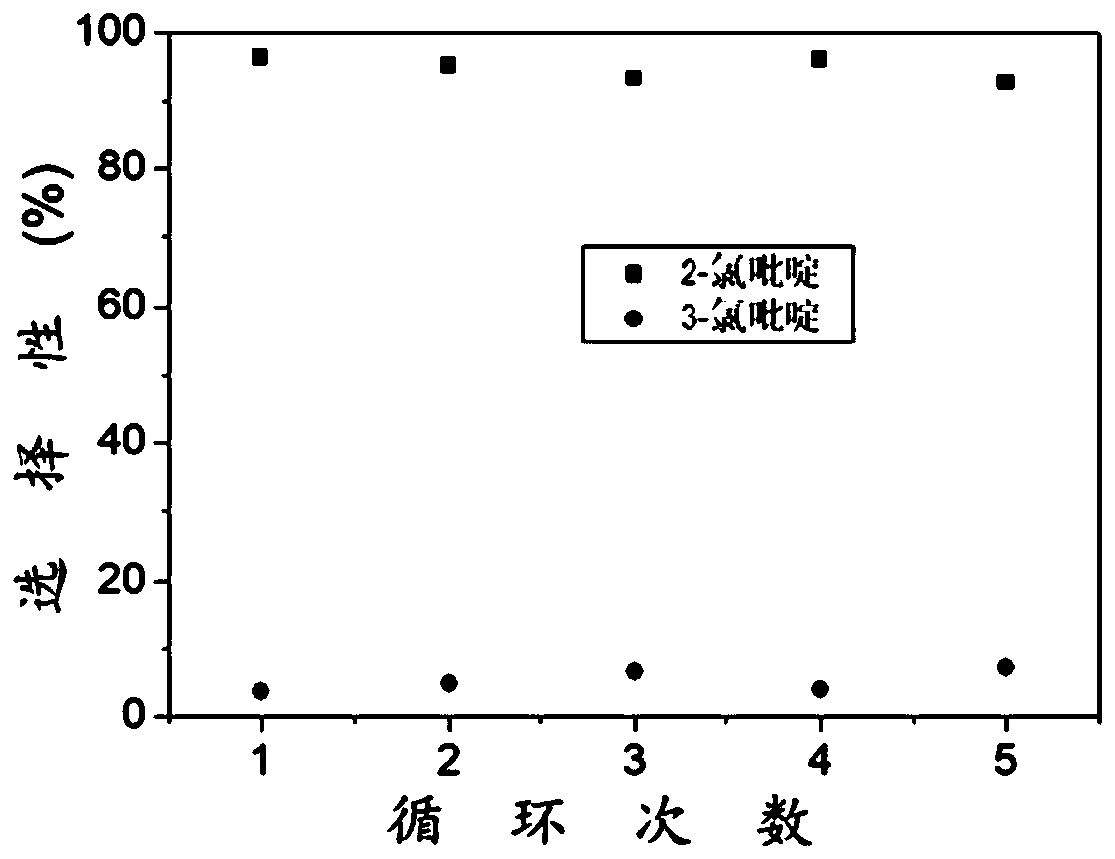
[0045] exist 1 Only the signal of the hydrogen atom corresponding to 2-chloropyridine was found in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com