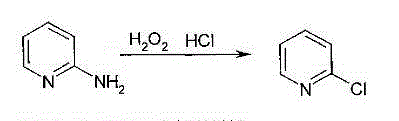
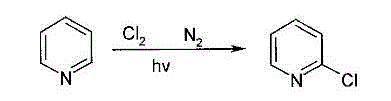
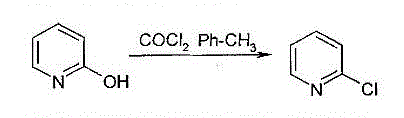Method for producing 2-chloropyridine and 2,6-dichloropyridine with inorganic solvent process
A technology of inorganic solvent and dichloropyridine, which is applied in the field of producing 2-chloropyridine and 2 by inorganic solvent method, can solve the problems of low economic benefit, by-products, and difficult source of 2-hydroxypyridine, and achieve high economic value and easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Pyridine and deionized water are mixed according to the mass ratio of 0.8:1 to prepare 20Kg of pyridine aqueous solution, and the pyridine aqueous solution is vaporized to make vaporized product; the vaporized product and chlorine gas are mixed according to the mass ratio of 1.5:1. The amount of feed is sent to the quartz reactor at a rate of 5Kg / h to make it react under the irradiation of an ultraviolet lamp. The reaction pressure is controlled at -0.3 to -0.5KPa, and the reaction temperature is controlled at 310-340°C. cooling to obtain the reaction solution; neutralize the pH value of the reaction solution to 10 with sodium hydroxide solution, then distill the reaction solution to obtain a mixed solution of organic matter and water; leave the mixed solution to separate layers, take out the organic phase, and then The organic phase was rectified under the condition of -0.08 to -0.085MPa, and the fractions in the temperature range of 110-113°C were collected to obtain 9...
Embodiment 2
[0032] Pyridine and deionized water are mixed according to the mass ratio of 0.3:1 to prepare 20Kg of pyridine aqueous solution, and the pyridine aqueous solution is vaporized to make vaporized product; the vaporized product and chlorine gas are mixed according to the mass ratio of 5:1 to obtain The amount of feed is sent to the quartz reactor at a rate of 8Kg / h to make it react under the irradiation of an ultraviolet lamp. The reaction pressure is controlled at -0.3 to -0.5KPa, and the reaction temperature is controlled at 260-290°C. cooling to obtain the reaction solution; neutralize the pH value of the reaction solution to 10 with sodium hydroxide solution, then distill the reaction solution to obtain a mixed solution of organic matter and water; leave the mixed solution to separate layers, take out the organic phase, and then The organic phase was rectified under the condition of -0.08 to -0.085MPa, and the fractions in the temperature range of 110-113°C were collected to o...
Embodiment 3
[0034] Pyridine and deionized water are mixed according to the mass ratio of 0.5: 1 to prepare 20Kg of pyridine aqueous solution, and the pyridine aqueous solution is vaporized to make vaporized product; the vaporized product and chlorine gas are mixed according to the mass ratio of 3: 1 to obtain The amount of feed is sent to the quartz reactor at a rate of 6Kg / h to make it react under the irradiation of an ultraviolet lamp. The reaction pressure is controlled at -0.3 to -0.5KPa, and the reaction temperature is controlled at 280-300°C. cooling to obtain the reaction solution; neutralize the pH value of the reaction solution to 10 with sodium hydroxide solution, then distill the reaction solution to obtain a mixed solution of organic matter and water; leave the mixed solution to separate layers, take out the organic phase, and then The organic phase was rectified under the condition of -0.08 to -0.085MPa, and the fractions in the temperature range of 110-113°C were collected to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com