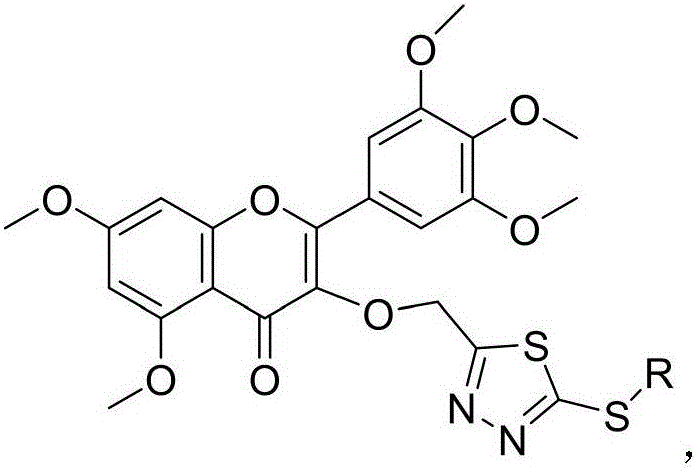
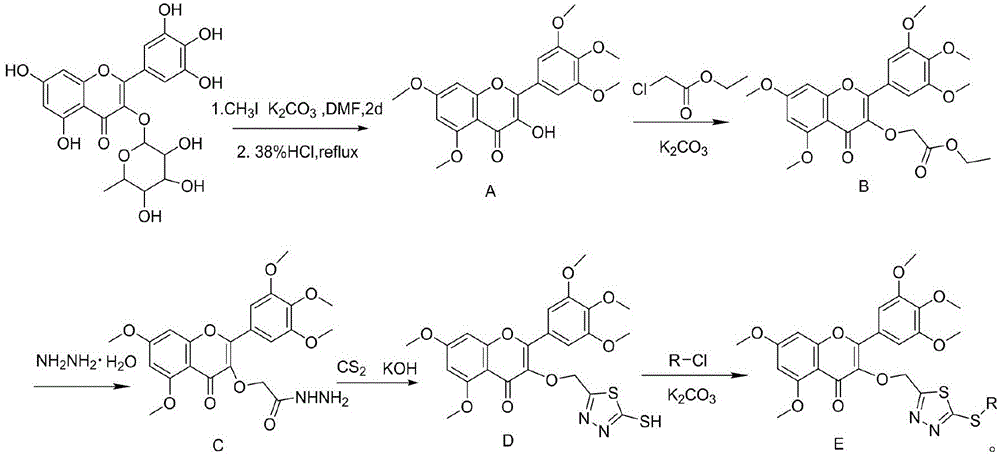
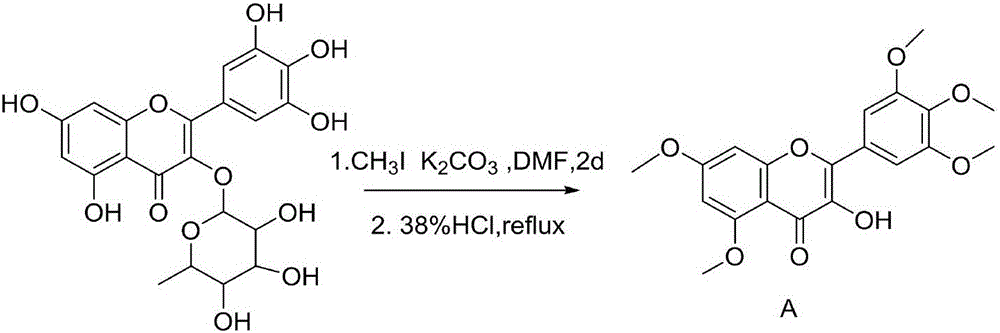Myricetin derivative containing thiadiazole thioether structure and preparation method thereof
A technology of thiadiazole sulfide and derivatives, which is applied to the application of inhibiting plant viruses, and the field of preparation of thiadiazole sulfide-based myricetin derivatives, which can solve the problem that myricetin does not involve plant viruses, myricetin and its derivatives. Problems such as less research on derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1, 3-((5-mercapto-1,3,4-thiadiazol-2-yl)methoxy)-5,7-dimethoxy-2-(3,4,5-trimethyl Oxyphenyl)-4H-chromen-4-one (compound number is III 1 ).
[0046] The first step: the preparation of 3-hydroxy-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (intermediate A);
[0047] In a 100mL round bottom flask, add 1.86g (4mmol) myricetin and dissolve it in 50mL DMF, add 8.86g (64mmol) K 2 CO 3 After stirring for 50min, slowly add 3mL (48mmol) methyl iodide, after completion, raise the temperature to 40°C, and stir at this temperature for 48h, filter the precipitate from the reaction system, wash with dichloromethane, combine the filtrate, pour the filtrate into 100mL water , extracted with dichloromethane (3×30mL), combined the organic phases, concentrated under reduced pressure, diluted the concentrate with 30mL of absolute ethanol, raised the temperature to reflux for 1h, after the solution was clarified, added 8mL of concentrated hydrochloric acid under reflu...
Embodiment 2
[0054] Example 2, 5,7-dimethoxy-3-((5-(methylthio)-1,3,4-thiadiazol-2-yl)methoxy)-2-(3,4 ,5-trimethoxyphenyl)-4H-chromen-4-one (compound number is III 2 ).
[0055] Intermediate D was obtained by the steps of Example 1. In a 50mL round-bottomed flask, add intermediate D (0.97mmol), anhydrous potassium carbonate (1.45mmol), and 30mL acetonitrile in sequence, stir at room temperature for 0.5h, and slowly add a solution of iodomethane (1.45mmol) in acetonitrile (10mL) dropwise , be warmed up to 45°C, continue to react at this temperature for 4-6h, follow the reaction by TLC (reaction system shows fluorescence under ultraviolet light, developer: dichloromethane:methanol=7:1, V / V), stop the reaction, After cooling to room temperature, a large amount of solids were precipitated, left to stand, filtered, and recrystallized from methanol to obtain the target compound with a yield of 52.2% and a melting point of 165.0-166.0°C.
Embodiment 3
[0056] Example 3, 5,7-dimethoxy-3-((5-((3-methylbenzyl)thio)-1,3,4-thiadiazol-2-yl)methoxy) -2-(3,4,5-trimethoxyphenyl)-4H-chromene-4-one (compound number is III 3 ).
[0057] Synthesized according to the conditions and methods of Example 2 to obtain 5,7-dimethoxy-3-((5-((3-methylbenzyl)thio)-1,3,4-thiadiazole-2 -yl)methoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one, yield 46.2%, melting point 105.0-106.0°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com