Functional 1,4,5,8-naphthalimide supermolecular organogel based on 4-aminopyridine, and application
A technology of naphthalimide and aminopyridine, which is applied in the field of ion detection and supramolecular materials, and can solve the problems of ion/molecular detection limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. Preparation of Supramolecular Polymer Organogel (ONT)
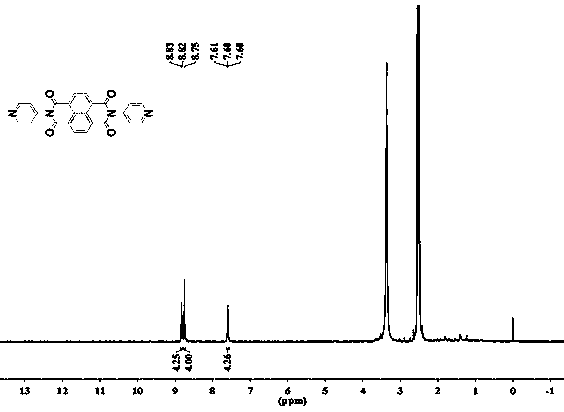
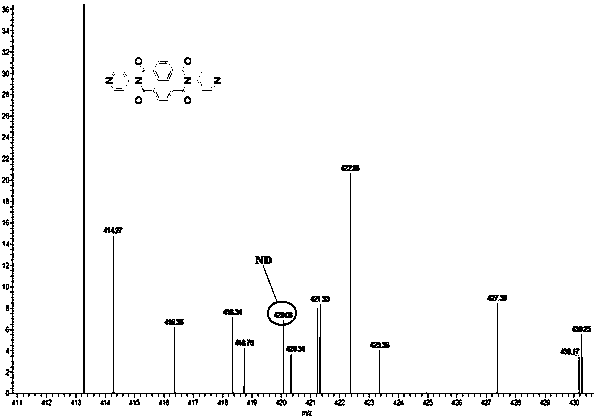
[0042] (1) Synthesis of ND: In 100ml of solvent DMF, add 2.68g (10mmol) 1,4,5,8-naphthalene tetracarboxylic anhydride and 1.88g (20mmol) 4-aminopyridine, N 2 Under protection, react at 140°C for 72 hours; after the reaction, spin out DMF with a rotary evaporator; wash the obtained black product with ethanol, recrystallize with DMSO and water, and dry to obtain 4-aminopyridine functionalized 1,4,5 , 8-naphthalene tetracarboximide supramolecular compound ND, the yield rate is 70%, and the melting point is greater than 250°C.
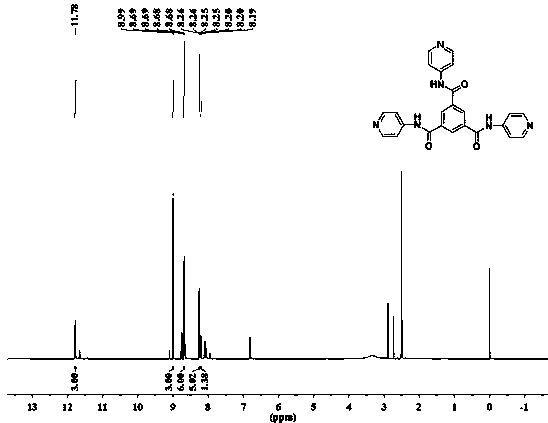
[0043] (2) Synthesis of TCP: In the solvent 30ml DMF, add 0.7528g (8mmol) 4-aminopyridine until it is completely dissolved, then add 1,3,5-trimethene dropwise into it with a constant pressure dropping funnel Acyl chloride (0.5278g, 2mmol, 2 drops in about 1 minute), reacted with triethylamine as an acid-binding agent (1.5mL) at room temperature for 12h; after the reaction was comple...
Embodiment 2
[0045] Embodiment 2, supramolecular organogel ONT to Fe 3+ 、Cu 2+ Efficient identification of
[0046] In the supramolecular polymer organogel ONT, add 2 times the equivalent of Mg 2+ , Ca 2+ , Cr 3+ , Fe 3+ ,Co 2+ , Ni 2+ , Cu 2+ , Zn 2+ , Ag + , Cd 2+ , Hg 2+ , Pb 2+ , Ba 2+ , Al 3+ , La 3+ and Eu 3+ (0.1M) solution, only the addition of Fe 3+ 、Cu 2+ , the fluorescence of ONT is quenched, and the addition of other cations cannot quench the fluorescence of ONT, so that the Fe 3+ 、Cu 2+ identified from numerous cations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com