Novel 4-aminopyridine and 6-aminopyrimidine carboxylates as herbicides
A C1-C6, alkyl technology, applied in the field of new 4-aminopyridine and 6-aminopyrimidine carboxylate as herbicides, can solve problems such as affecting crop yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0186] Compound preparation method
[0187] Exemplary methods for the synthesis of compounds of formula (I) are provided below.
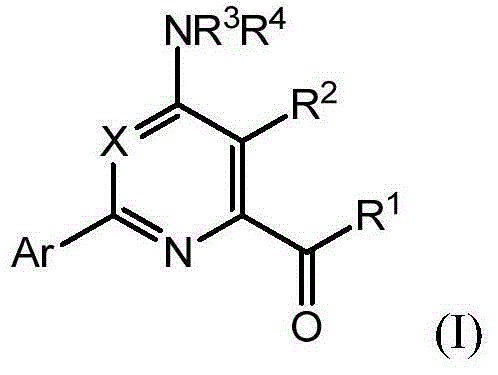
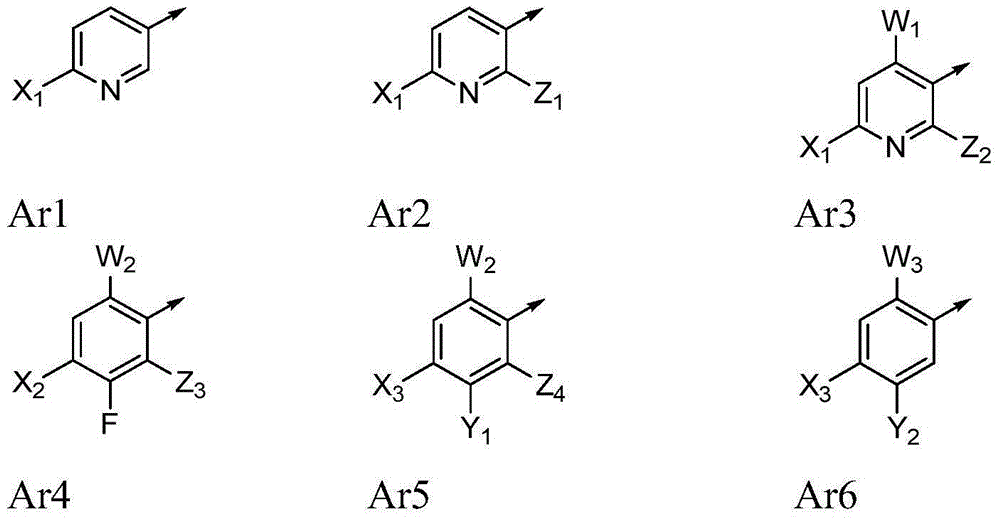
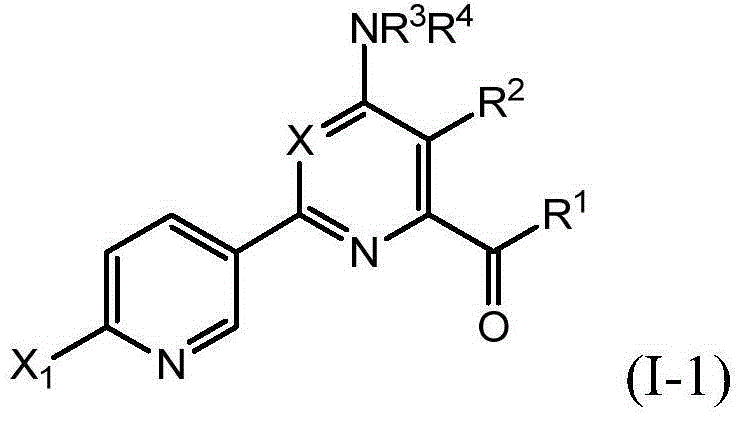
[0188] 3,5-Disubstituted-4-amino-6-(optionally substituted phenyl or pyridyl)pyridine-2-carboxylic acids of formula (I) can be prepared in various ways. As shown in Scheme I, 4-amino-6-chloro-pyridine-2-carboxylates of formula (II) can be prepared via Suzuki coupling with boronic acids or esters in a base such as potassium fluoride and a catalyst such as bis(tris Phenylphosphine)-palladium(II) dichloride in the presence of a polar protic solvent mixture such as acetonitrile-water at a temperature of for example 110° C., for example, in a microwave reactor into the 4-amino- 6-substituted-pyridine-2-carboxylates, wherein Ar is as defined herein (reaction a 1 ). 4-Amino-6-substituted-pyridine-2-carboxylates of formula (III) can be converted to phosphonates of formula (IV) via reaction with iodinating reagents such as periodic acid and iodine in a po...
Embodiment 1
[0229] Example 1: Preparation of 4-amino-3,6-dichloro-pyridine-2-carboxylic acid methyl ester (head A)
[0230]
[0231] Prepared as described in Fields et al., WO2001051468A1.
Embodiment 2
[0232] Example 2: Preparation of 4-amino-3,6-dichloro-5-fluoro-pyridine-2-carboxylic acid methyl ester (head B)
[0233]
[0234] Prepared as described in Fields et al., Tetrahedron Letters 2010, 51, 79-81.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com