Tripodia pseudorotaxane supramolecular gel based on trimesoyl chloride and preparation and application of metal gel
A technology of trimesoyl chloride and supramolecular gel, which is applied in gel preparation, analytical materials, colloid chemistry, etc., and can solve the problems of complicated operation, expensive equipment, and unsuitable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、 3
[0052] Embodiment 1, the synthesis of tripod pseudorotaxane supramolecular organogel TP-Q
[0053] (1) Synthesis of pillar[5]arene TP: see T. Ogoshi, S. Kanai, S. Fujinami, T.Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 for details;
[0054] (2) Synthesis of 4-aminopyridine-functionalized trimesoyl chloride gelling factor Q: see X.Z. Luo, X. J. Jia, J. H. Deng, J. L. Zhong, H. J. Liu, K. J. Wang, and D. C.Zhong, J. Am. Chem. Soc., 2013, 135, 11684-11687;
[0055] (3) Synthesis of tripod pseudorotaxane supramolecular organogel (TP-Q): Weigh 4-aminopyridine-functionalized trimesoyl chloride gel factor Q (4.1mg, 0.0093mmol) and put it on the column[5] Aromatic TP (20.2 mg, 0.027 mmol), added to 0.5 mL DMSO-HO 2 O (0.3 mL DMSO, 0.2 mL H 2 O), fully dissolved under heating to obtain a transparent solution; cooled to room temperature, the transparent solution formed a stable supramolecular organogel TP-Q.
Embodiment 2
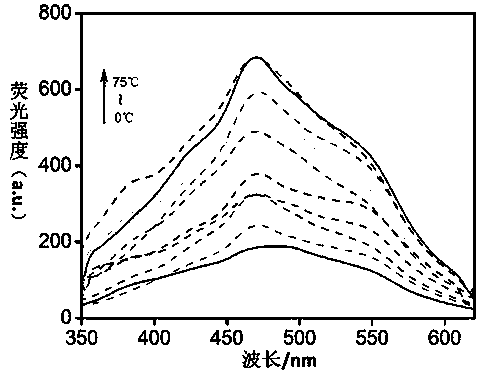
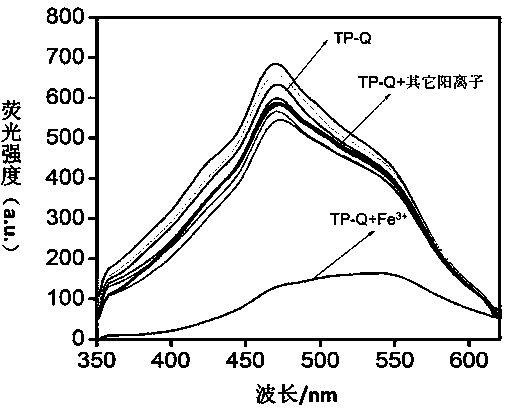
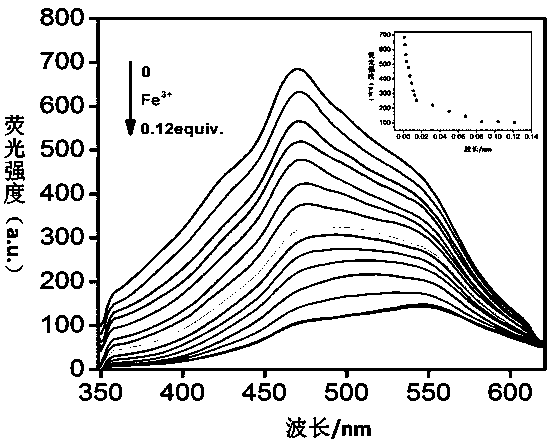
[0056] Embodiment 2, TP-Q fluorescent recognition Fe 3+
[0057] Take 13 small amounts (about 0.02g) of organogel TP-Q on a white drip plate, and add 20 μL of different cations (C=0.1moL / L, Mg 2+ , Ca 2+ , Cr 3+ , Fe 3+ ,Co 2+ , Ni 2+ , Cu 2+ , Zn 2+ , Ag + , Cd 2+ , Hg 2+ , Pb 2+ ) in aqueous solution. Observe the fluorescence color change under the ultraviolet light. If the fluorescence color of organogel TP-Q changes from blue-white to black, it means that Fe is added. 3+ Solution, if the fluorescent color of TP-Q does not change, it means that the aqueous solution of other cations is added.
Embodiment 3
[0058] Embodiment 3, supramolecular metal gel TP-Q+Fe 3+ preparation of
[0059] Weigh column [5] arene TP (20.2 mg, 0.027 mmol), 4-aminopyridine functionalized trimesoyl chloride gelling factor Q (4.1 mg, 0.0093 mmol) and ferric perchlorate hexahydrate (4.6 mg, 0.010 mmol), added together to 0.5mL DMSO-H 2 O (0.3 mL DMSO, 0.2 mL H 2 O), heated to dissolve it, and cooled to room temperature to form a supramolecular organogel based on tripod quasirotaxane (TP-Q+Fe 3+ ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com