Carbamide-containing acetylated chitosan quaternary ammonium salt, and preparation method and application thereof
A technology of acetylated chitosan and chitosan, which is applied in the field of daily chemicals, can solve the problems of poor water solubility and affecting the range of use of chitosan, and achieve the effects of improved biological activity, easy availability of equipment and raw materials, and easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthetic route of urea-containing acetylated chitosan quaternary ammonium salt is as follows.
[0037]
[0038] Wherein, R is chlorophenyl or phenyl; the average degree of polymerization n ranges from 20 to 3000.
[0039] In this example, the quaternary ammonium salt of the target compound containing urea-containing acetylated chitosan was synthesized according to the above synthetic route.
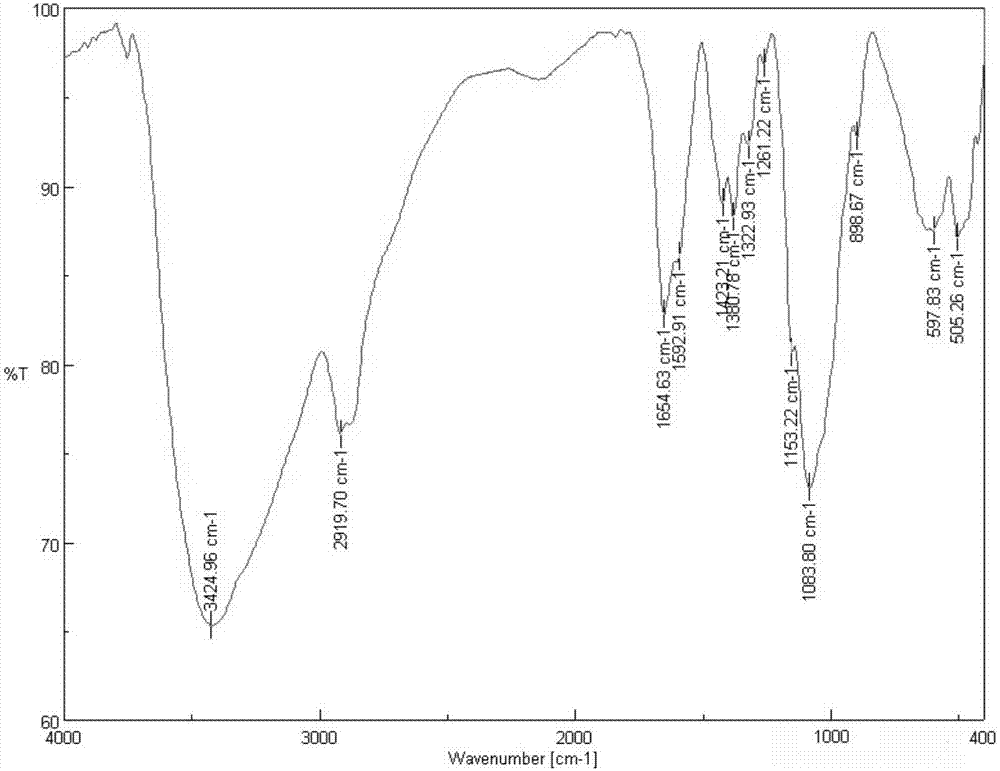
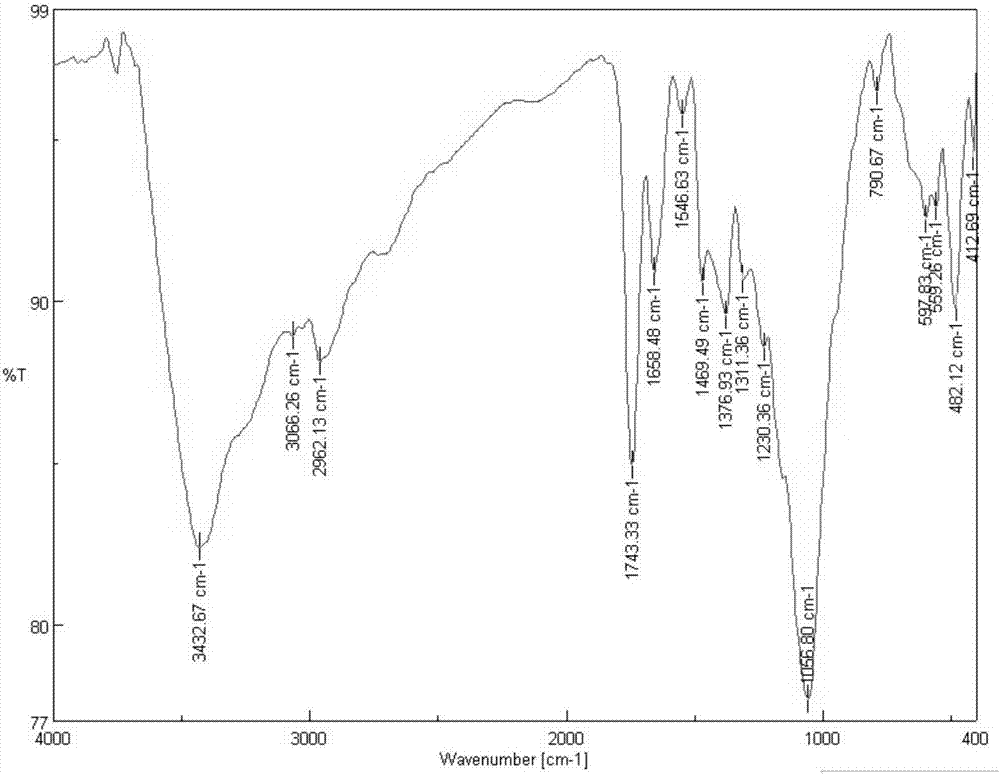
[0040] 1) Preparation of chloroacetylated chitosan quaternary ammonium salt: 1.61g chitosan (see figure 1 ) in 80mL of 1-methyl-2-pyrrolidone, add 4.5g of sodium iodide, 15mL of 15% sodium hydroxide solution and 15mL of methyl iodide successively under stirring, reflux and stir at 60°C for 2h, and wait for the reaction After the end, add 1.51mL chloroacetyl chloride dropwise, react at room temperature for 24h, then precipitate with 250mL dehydrated alcohol, after suction filtration, washing, and vacuum freeze-drying, the product chloroacetylated chitosan quaternary ammoniu...
Embodiment 2
[0044] The difference from Example 1 is:
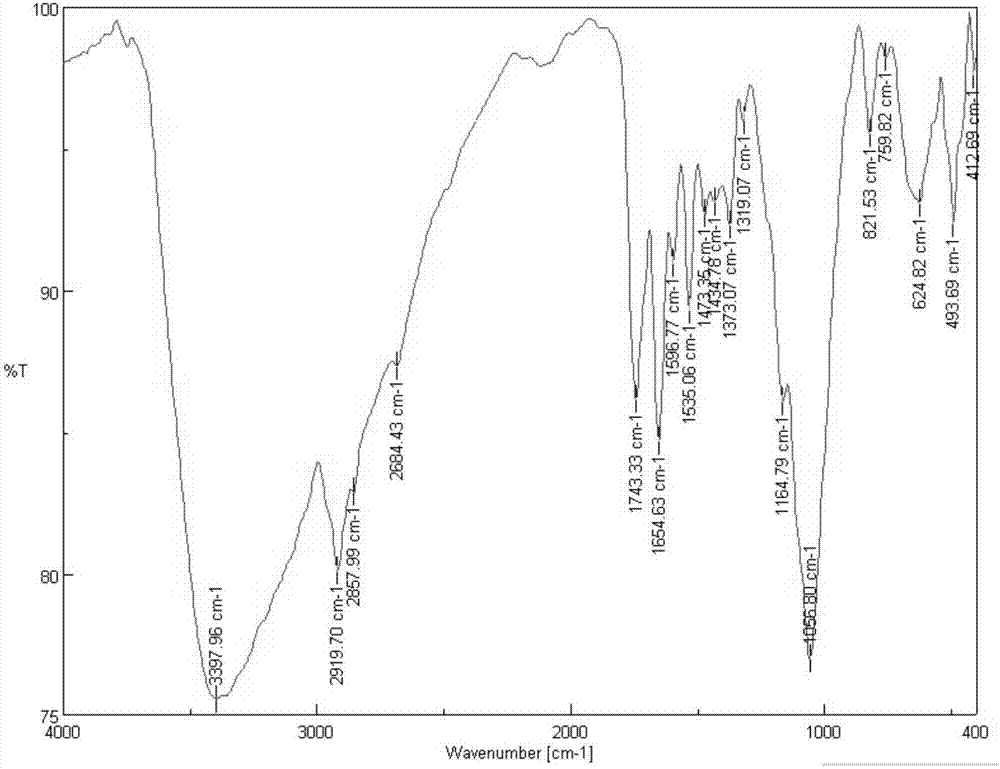
[0045] 1) Preparation of chloroacetylated chitosan quaternary ammonium salt: 1.61g chitosan (see figure 1 ) was dispersed in 80mL of 1-methyl-2-pyrrolidone, and 4.5g of sodium iodide, 15mL of 15% sodium hydroxide solution and 15mL of methyl iodide were added successively under stirring, and stirred under reflux at 60°C for 4h. After the end, add 2.265mL chloroacetyl chloride dropwise, react at room temperature for 24h, then precipitate with 300mL dehydrated alcohol, after suction filtration, washing, and vacuum freeze-drying, the product chloroacetylated chitosan quaternary ammonium salt (see figure 2 ) 3.7g, set aside.
[0046] 2) Preparation of urea: Dissolve 1.63g of triphosgene in 5mL of ethyl acetate, add dropwise 6mL of ethyl acetate solution with a concentration of 2mmol / mL 2-chloroaniline into it under ice bath, stir at room temperature for 1h, and heat at 60°C Reflux to clarification, then suspend distillation under reduce...
Embodiment 3
[0049] The difference from Example 1 is:
[0050] 1) Preparation of chloroacetylated chitosan quaternary ammonium salt: 1.61g chitosan (see figure 1 ) in 80mL of 1-methyl-2-pyrrolidone, add 4.5g of sodium iodide, 15mL of 15% sodium hydroxide solution and 15mL of methyl iodide successively under stirring, reflux and stir at 60°C for 2h, and wait for the reaction After the end, add 1.51mL chloroacetyl chloride dropwise, react at room temperature for 36h, then precipitate with 250mL dehydrated alcohol, after suction filtration, washing, and vacuum freeze-drying, the product chloroacetylated chitosan quaternary ammonium salt (see figure 2 )3.4g, for use.
[0051] 2) Preparation of urea: Dissolve 1.63g of triphosgene in 5mL of ethyl acetate, add dropwise 5mL of ethyl acetate solution with a concentration of 2mmol / mL 3-chloroaniline in an ice bath, stir at room temperature for 2h, and then dissolve at 70°C Reflux to clarification, then suspend distillation under reduced pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com