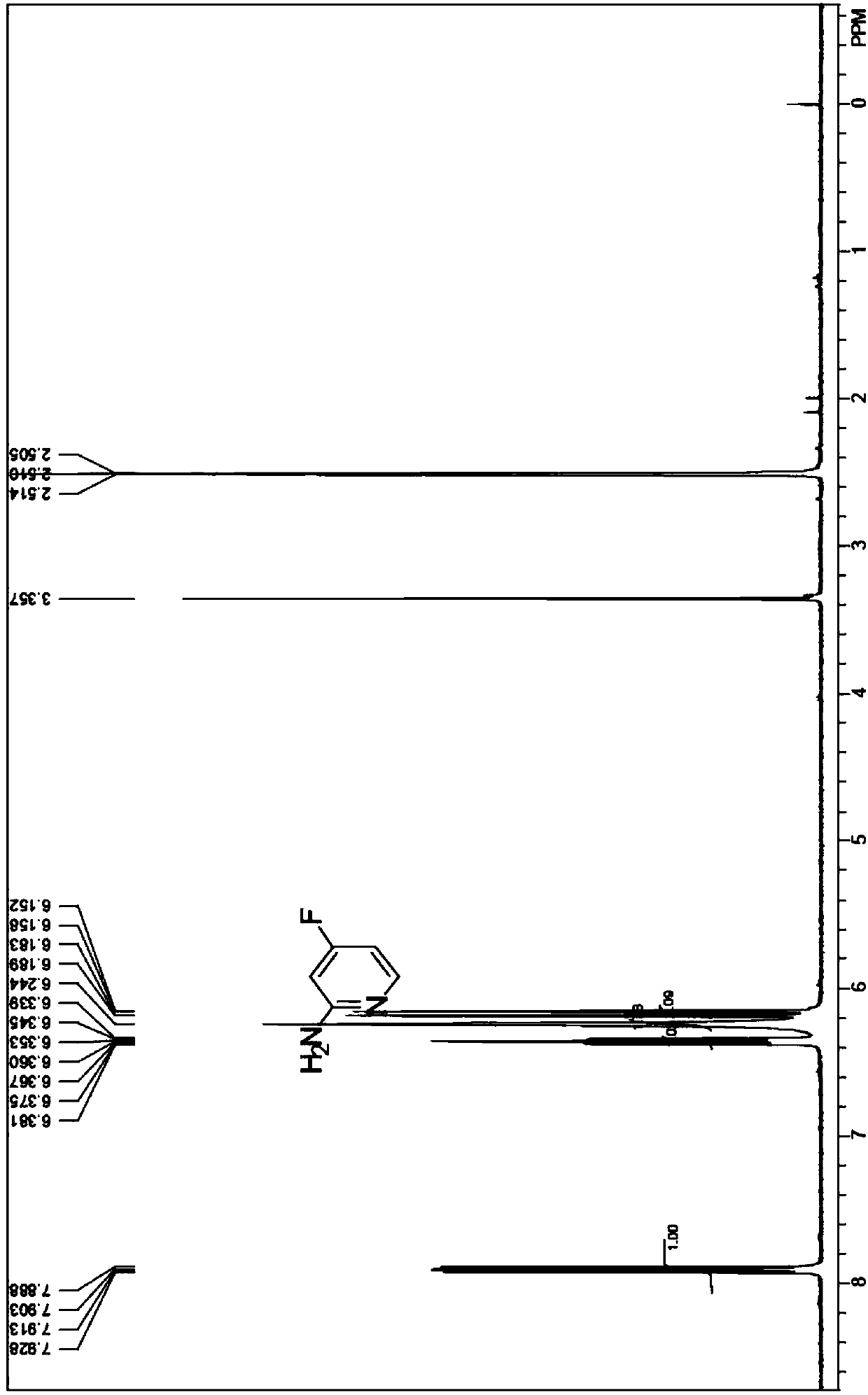
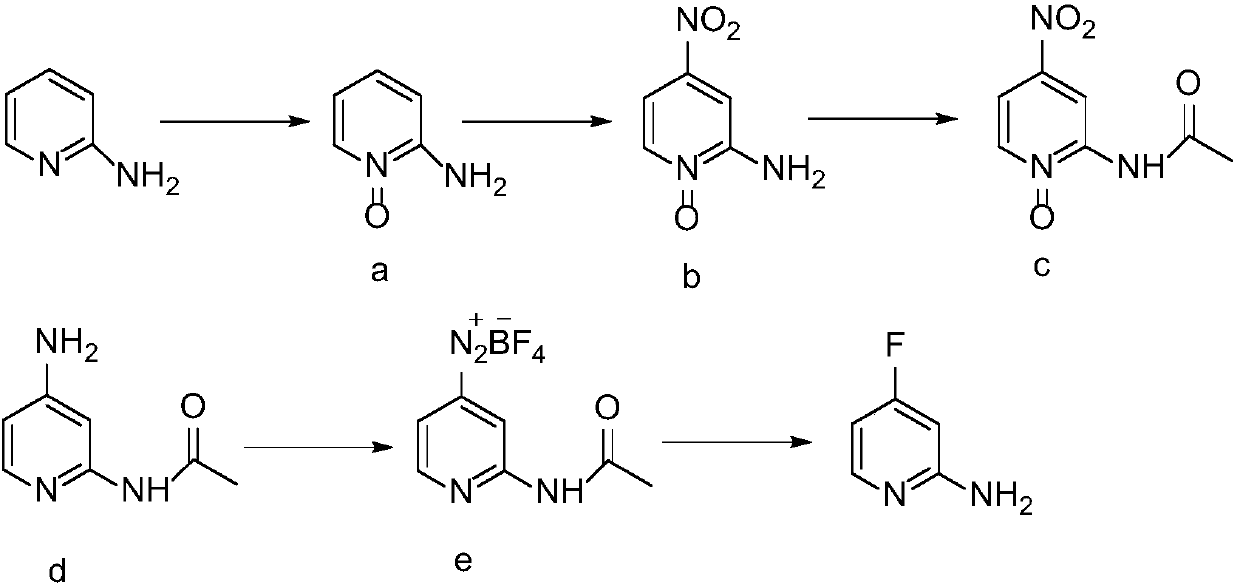
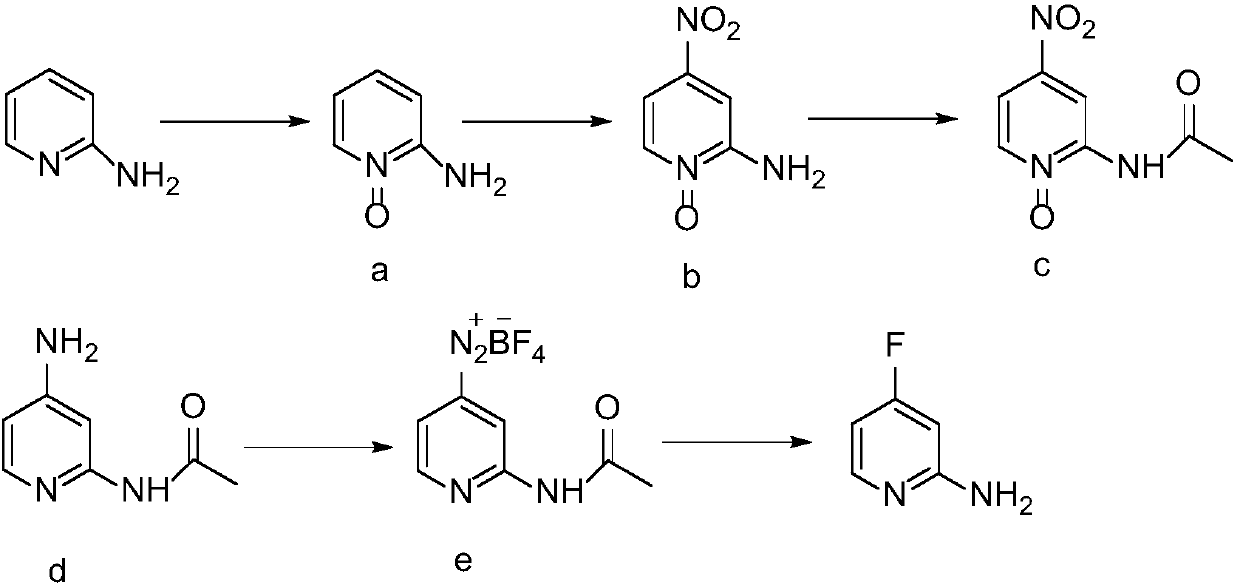
Synthesis method of 2-amino-4-fluoropyridine
A synthetic method, fluoropyridine technology, applied in the direction of organic chemistry, etc., can solve the problems of complex operation, low yield, serious pollution, etc., and achieve the effect of simple operation process and cheap and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] 1) Preparation of 2-aminopyridine N-oxide
[0027] Acetic acid (220mL) and acetic anhydride (110mL) were added into a 1L four-necked flask, and raw material diaminopyridine (94.2g, 1.0mol) and hydrogen peroxide (200ml, 1.9mol) with a mass concentration of 30% were added successively under stirring at room temperature, and the Stir at 75°C for 3h, add acetic anhydride (50ml) and 30% hydrogen peroxide (90ml, 0.9mol), and continue the reaction for 3h. After the reaction, the solvent was distilled off under reduced pressure, and the residue was cooled to room temperature, and adjusted to pH = 7-8 with saturated sodium carbonate solution. Extract with chloroform (100mL×3), and recover the solvent in the organic phase under reduced pressure at low temperature to obtain an orange-red liquid containing compound a, which is directly used in the next reaction without purification. The reaction formula is as follows:
[0028]
[0029] 2) Preparation of 2-amino-4-nitropyridine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com