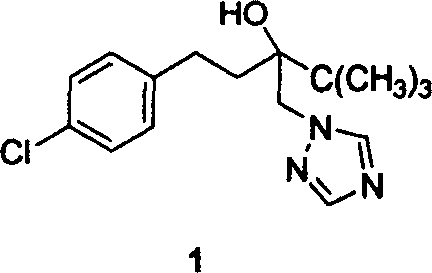
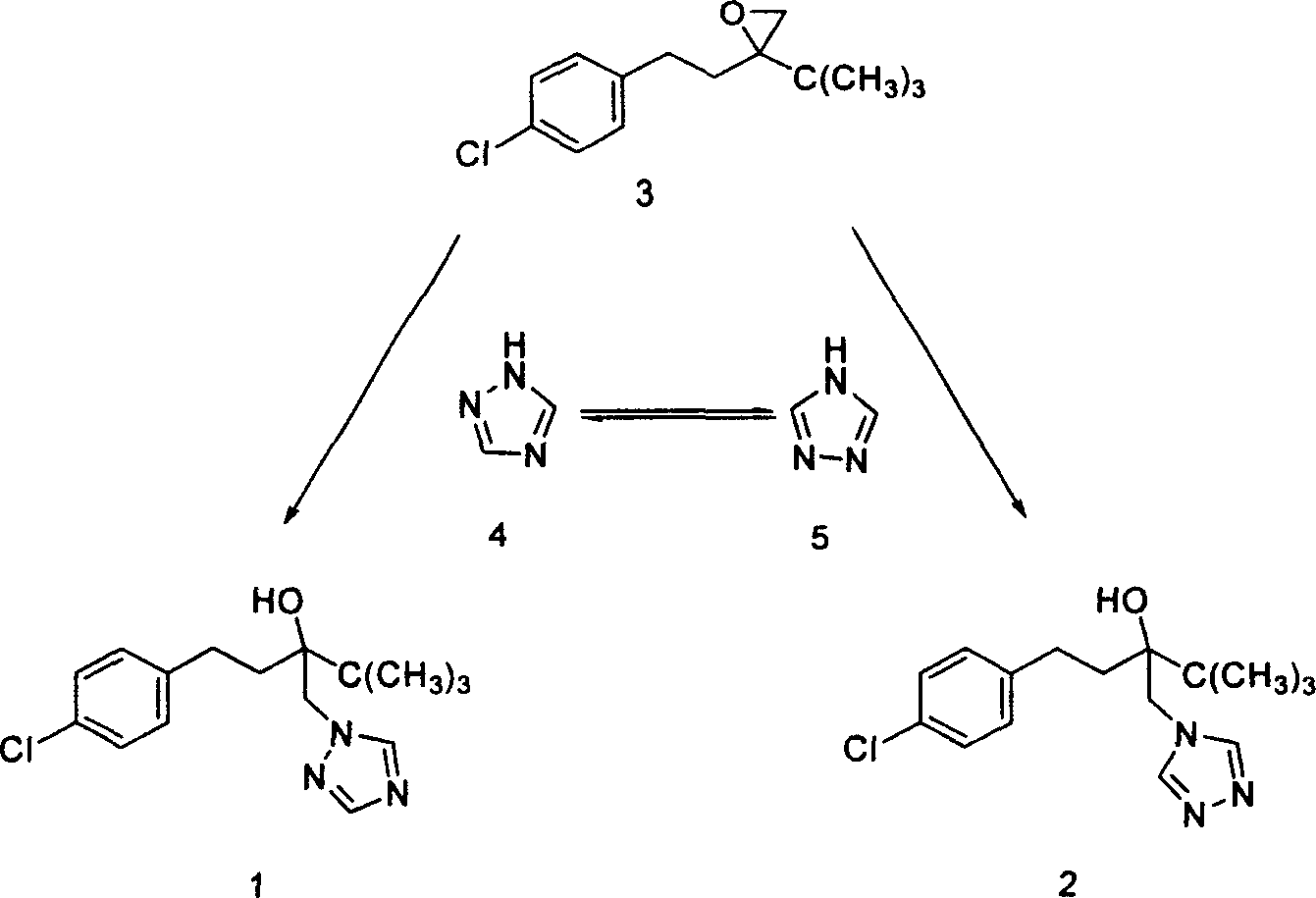
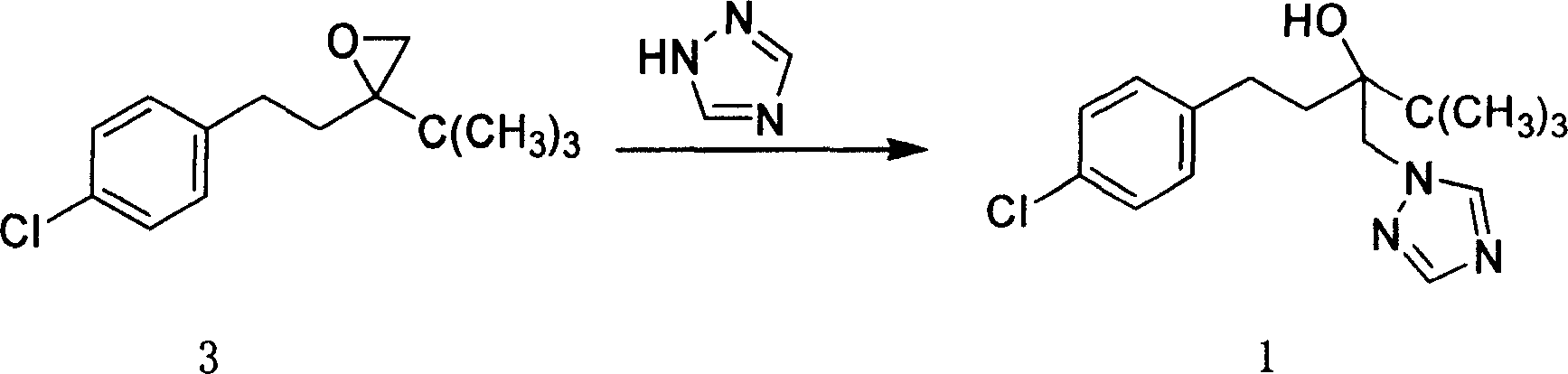
Method for preparing Tebucomazole in high purity
A technology of purity tebuconazole and chlorophenethyl is applied in the synthesis field of pesticide tebuconazole-1--3-pentanol), and can solve the problems of difficult separation, yield lower than 76%, no bactericidal activity and the like , to achieve the effect of convenient operation, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 120g (0.40mol)3, 3g (0.05mol) of potassium hydroxide, 40g (0.55mol) of 1,2,4-triazole, 1g of N,N-dimethyl-4-aminopyridine, and 100mL of n-butanol A 500mL three-necked flask with a stirring and reflux tube was stirred for 6 hours at a reflux temperature of 120°C. After the reaction was complete, hydrochloric acid was added for neutralization and phase separation. The organic phase was cooled and crystallized, filtered and dried to obtain 102g of a white solid, w(1)≥98.0% (liquid Phase chromatography, external standard), yield ≥ 81.4% (based on 3). Melting point 102-104°C (literature value: melting point 102-104°C).
[0027] The spectrogram data of tebuconazole 1 is as follows ESI-MS (m / z): 308 (M + ). 1 H NMR (400MHz, CDCl 3 ), δ: 1.03(s, 9H, C(CH 3 ) 3 ), 1.67 (tt, J = 14.0Hz, 4.0Hz, 1H, CH 2 ), 1.82 (m, 2H, CH 2 CH 2 ), 2.44(tt, J=14.0Hz, 4.0Hz, 1H, CH 2 ), 3.01(s, 1H, OH), 4.33(d, J=14.4Hz, 1H, CH 2 ), 4.38 (d, J=14.4Hz, 1H, CH 2 ), 6.95 (d, J=8.4Hz, 2H...
Embodiment 2
[0029] Add 120g (0.40mol)3, 6g (0.10mol) of potassium hydroxide, 40g (0.55mol) of 1,2,4-triazole, 1g of N,N-dimethylaniline, and 100mL of dimethylformamide with stirring and a 500mL three-neck flask with a reflux tube, stirred at 130°C for 4h, after the reaction was complete, added hydrochloric acid to neutralize, and the solvent was recovered by distillation under sub-pressure, the organic phase was cooled and crystallized, washed with water, filtered and dried to obtain 106g of a white solid, w(1)≥98.6% (liquid Phase chromatography, external standard), the yield ≥ 85.0% (calculated as 3).
Embodiment 3
[0031] 120g (0.40mol)3, 7g (0.10mol) of sodium ethoxide, 40g (0.55mol) of 1,2,4-triazole, 0.5g of N,N-dimethyl-4-aminopyridine, and 100mL of n-butanol were added to the A 500mL three-neck flask with a stirring and reflux tube was stirred at 120°C for 6h. After the reaction was completed, hydrochloric acid was added to neutralize, and the solvent was recovered by distillation under secondary pressure. The organic phase was cooled and crystallized, filtered and dried to obtain 101g of a white solid, w (1) ≥ 98.3% ( liquid chromatography, external standard), the yield ≥ 80.7% (calculated as 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com