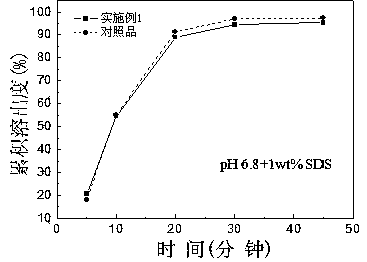
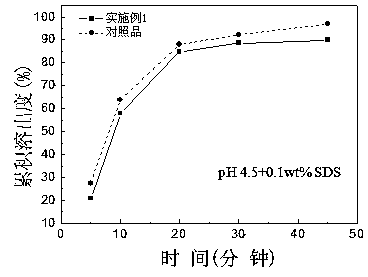
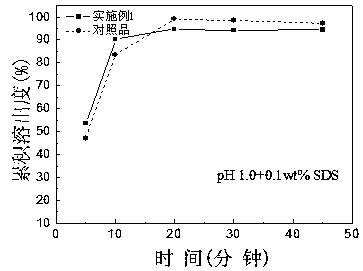
Roflumilast tablet and preparation method thereof
A technology of roflumilast tablets and excipients, which is applied in the field of roflumilast tablets and its preparation, can solve problems such as poor content uniformity of tablets, and achieve high product yield, easy industrialization, The effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The roflumilast pharmaceutical composition described in every 100 tablets, its formula composition is as shown in table 1 and table 2:
[0041] Table 1
[0042] .
[0043] Table 2
[0044] .
[0045] Process operation:
[0046] (1) Dissolve the raw material drug with absolute ethanol to form a raw material drug solution;
[0047] (2) PVP-K90 is crushed through a 100-mesh sieve, starch, lactose, and pregelatinized starch are passed through a 80-mesh sieve, the above four excipients are mixed evenly, and the ethanol solution of the raw material drug is added to make granules, dried, and granulated;
[0048] (3) Add magnesium stearate, mix evenly, and test the intermediate;
[0049] (4) According to the test data of the intermediate, determine the weight of the tablet, press the tablet, and the hardness of the tablet is greater than 12N;
[0050] (5) Coat the film with water-soluble water-containing material, and control the weight gain of the film coat to 2.5-3....
Embodiment 2
[0052] The roflumilast pharmaceutical composition described in every 100, its formula composition is as shown in table 3 and table 4:
[0053] table 3
[0054] .
[0055] Table 4
[0056] .
[0057] Process operation:
[0058] (1) Dissolve the raw material drug with absolute ethanol to form a raw material drug solution;
[0059](2) PVP-K90 is crushed through a 100-mesh sieve, starch, lactose, and pregelatinized starch are passed through a 80-mesh sieve, the above four excipients are mixed evenly, and the ethanol solution of the raw material drug is added to make granules, dried, and granulated;
[0060] (3) Add magnesium stearate, mix evenly, and test the intermediate;
[0061] (4) According to the test data of the intermediate, determine the weight of the tablet, press the tablet, and the hardness of the tablet is greater than 12N;
[0062] (5) Coat the film with water-soluble water-containing material, and control the weight gain of the film coat to 2.5-3.5%.
Embodiment 3
[0064] The roflumilast pharmaceutical composition described in every 100 tablets, its formula composition is as shown in table 5 and table 6:
[0065] table 5
[0066] .
[0067] Table 6
[0068] .
[0069] Process operation:
[0070] (1) Dissolve the raw material drug with absolute ethanol to form a raw material drug solution;
[0071] (2) PVP-K90 is crushed through a 100-mesh sieve, starch, lactose, and pregelatinized starch are passed through a 80-mesh sieve, the above four excipients are mixed evenly, and the ethanol solution of the raw material drug is added to make granules, dried, and granulated;
[0072] (3) Add magnesium stearate, mix evenly, and test the intermediate;
[0073] (4) According to the test data of the intermediate, determine the weight of the tablet, press the tablet, and the hardness of the tablet is greater than 12N;
[0074] (5) Coat the film with water-soluble water-containing material, and control the weight gain of the film coat to 2.5-3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com