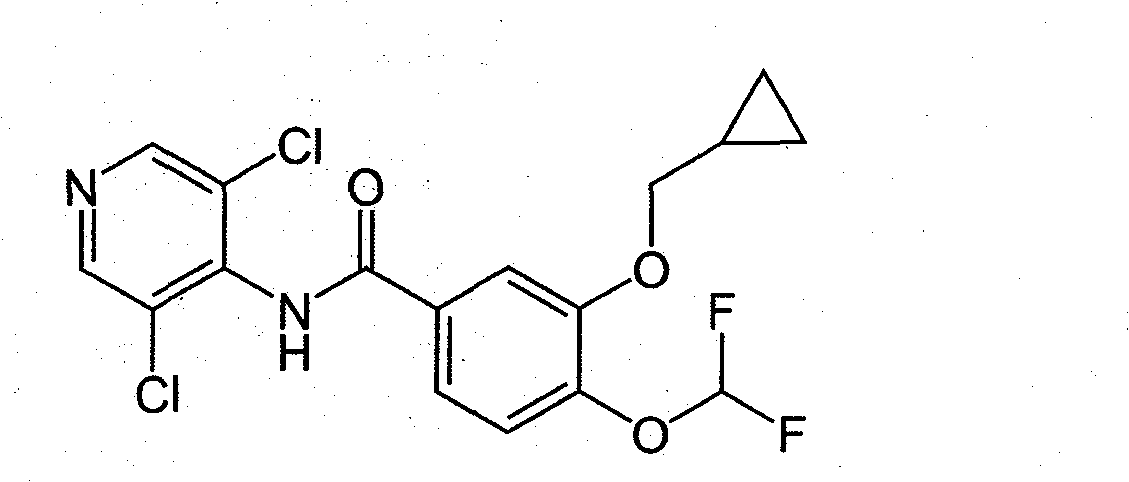
Roflumilast oral preparation and preparation method thereof
A technology for roflumilast and oral preparations, applied in the field of medicine, can solve the problems of unfavorable industrialization, increased drug cost for patients, and high input cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 0.5 g of roflumilast raw material, dissolve it in 80 ml of methanol, and use it as a raw material solution; weigh about 12 g of starch, and prepare 10% starch slurry by boiling paddle method, as a binder. Weigh 160g of lactose and 30g of microcrystalline cellulose after passing through an 80-mesh sieve, add them to a tank mixer, stir to mix evenly, then add roflumilast solution, stir to moisten evenly, dry at 85°C, and evaporate methanol to dryness. Take the mixture after evaporating methanol, put it in a trough mixer, add 55g of 15% starch slurry, mix to make soft materials, and granulate with a swinging granulator. Dry at 80°C, add 2 g of croscarmellose sodium and 1.5 g of magnesium stearate after sizing, and mix. Check the granule content, determine the weight of the tablet, compress the tablet, coat it with a film, pack it, and get the Roflumilast tablet.
Embodiment 2
[0025] Weigh 0.5g of roflumilast raw material and dissolve it in 70ml of 95% ethanol as a raw material solution; weigh about 15g of starch, and make 15% starch slurry by boiling paddle method as a binder. Weigh 130g of lactose and 60g of starch after passing through an 80-mesh sieve, add them to a tank mixer, stir to mix evenly, then add roflumilast solution, stir to moisten evenly, dry at 70°C, and evaporate ethanol to dryness. Take the mixture after evaporating ethanol, put it in a trough mixer, add 70 g of 15% starch slurry, mix to make soft materials, and granulate with a swinging granulator. Dry at 70°C, add 2g of magnesium stearate after granulation, and mix. Check the granule content, determine the weight of the tablet, compress the tablet, coat it with a film, pack it, and get the Roflumilast tablet.
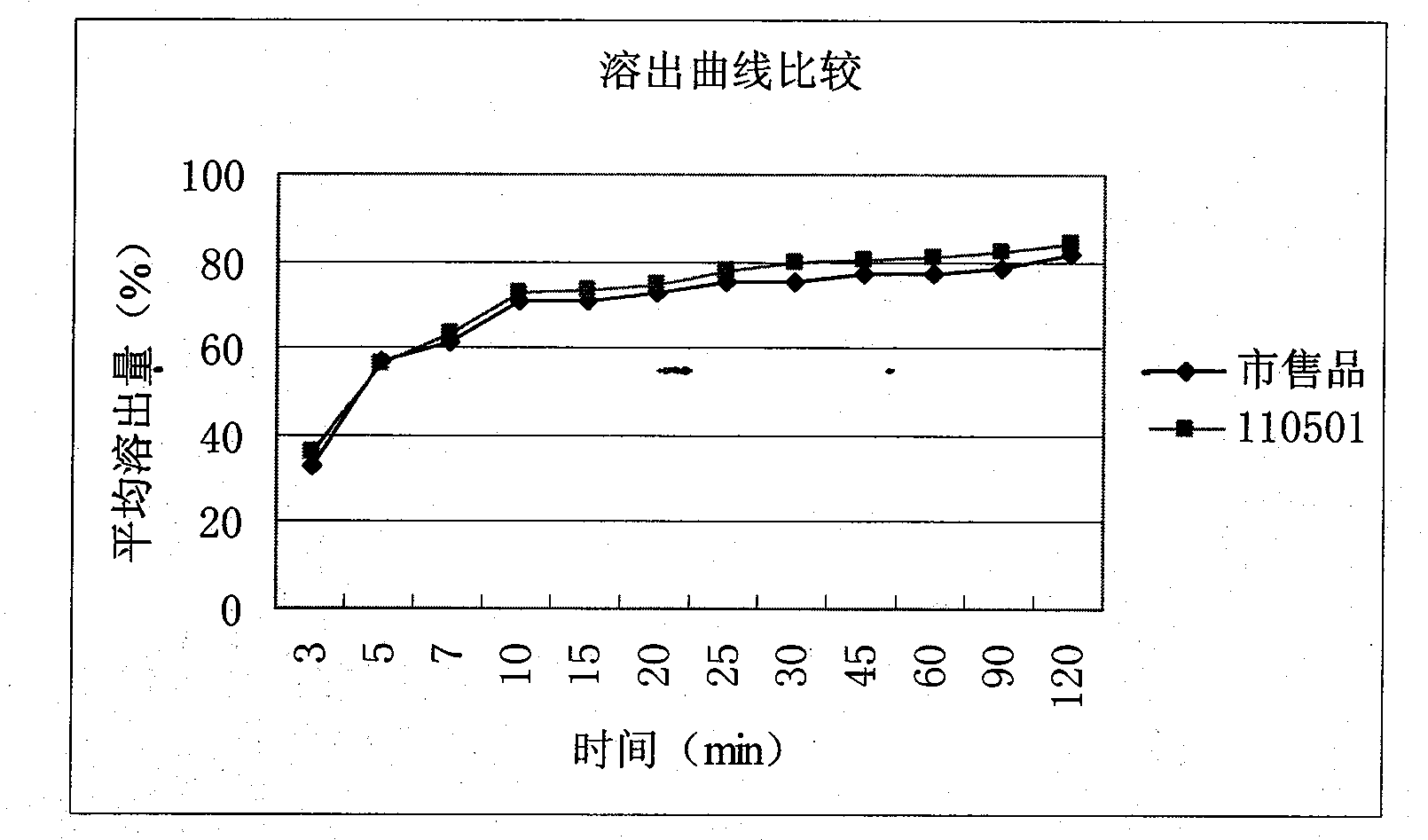
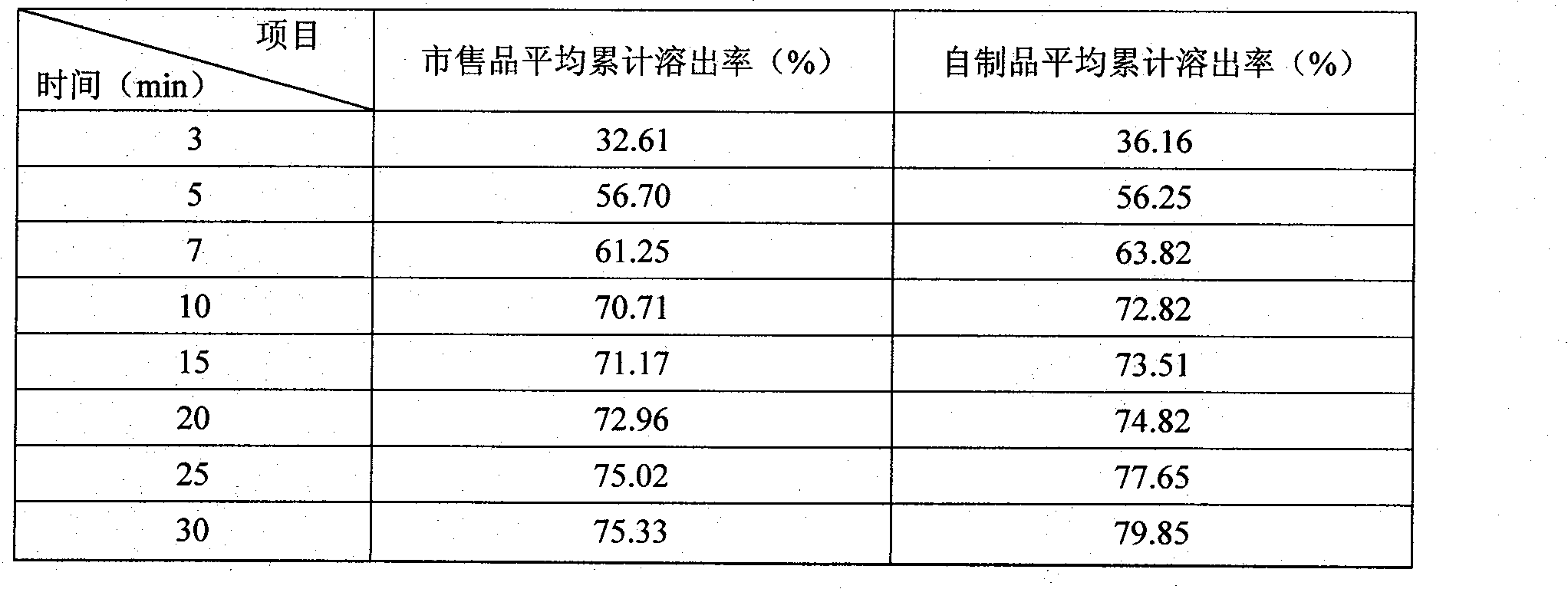
[0026] According to the method of Example 2, roflumilast tablets were prepared, the specification was 0.5 mg, and the batch number was 110501. Carried out the comparis...
Embodiment 3
[0032] Weigh 1.0 g of roflumilast raw material and dissolve it in 95 ml of 95% ethanol as a raw material solution; weigh about 6 g of PVPK30 to make a 6% PVPK30 solution as a binder. Weigh 185g of mannitol and 15g of hydroxypropyl cellulose after passing through a 100-mesh sieve, add them to a tank mixer, stir to mix evenly, then add roflumilast solution, stir to moisten evenly, dry at 70°C, and evaporate ethanol to dryness . Take the mixture after evaporating ethanol, put it in a trough mixer, add 65g of 6% starch slurry, mix to make soft materials, and granulate with a swinging granulator. Dry at 75°C, add 2g of micro-powder silica gel after sizing, and mix. Check the granule content, determine the weight of the tablet, compress the tablet, coat it with a film, pack it, and get the Roflumilast tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com