Novel crystalline state of roflumilast and preparation method thereof
A roflumilast, crystalline technology, used in the pharmaceutical field, can solve the problems of difficult removal, failure to meet drug quality standards, and affecting drug bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
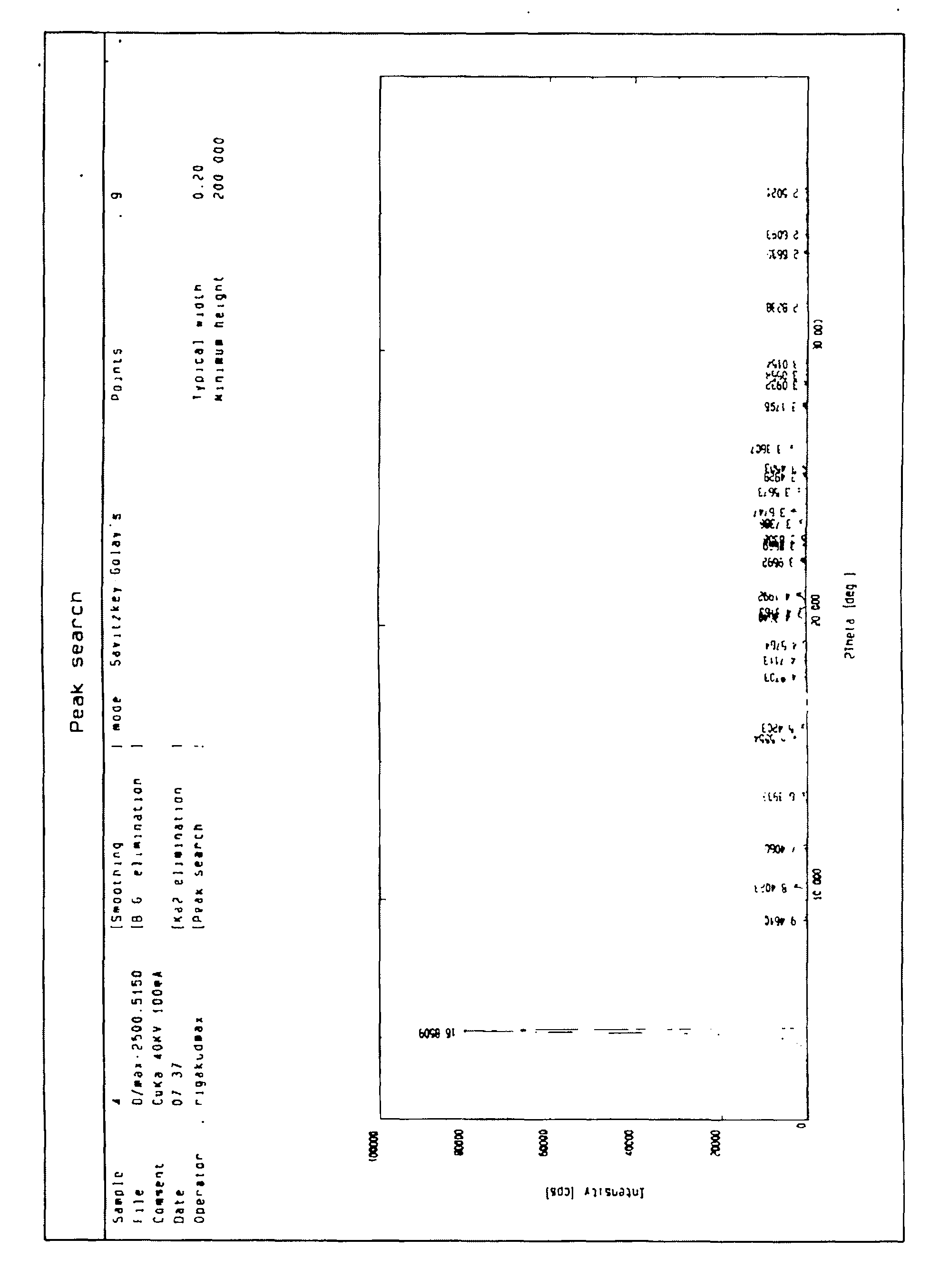
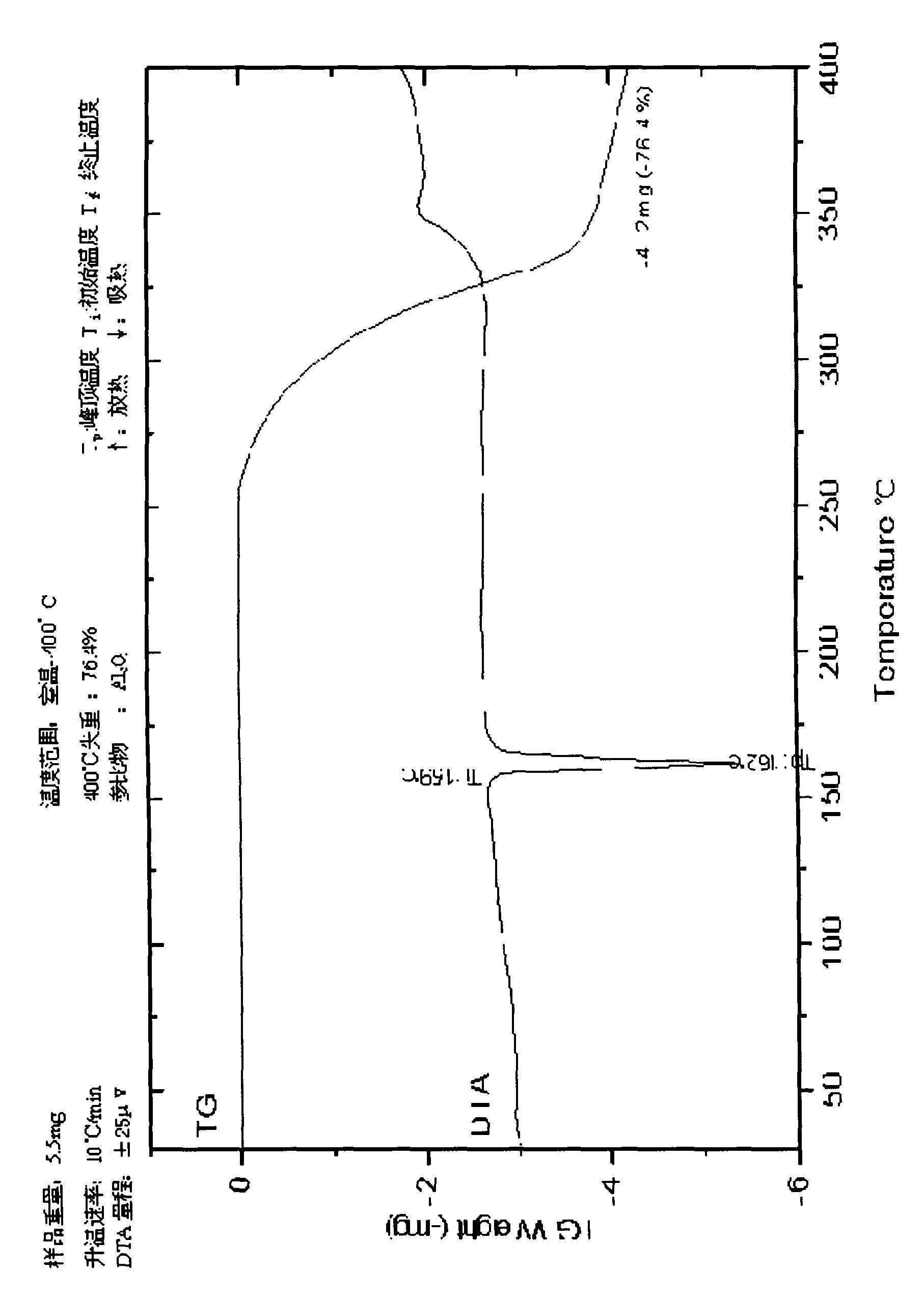
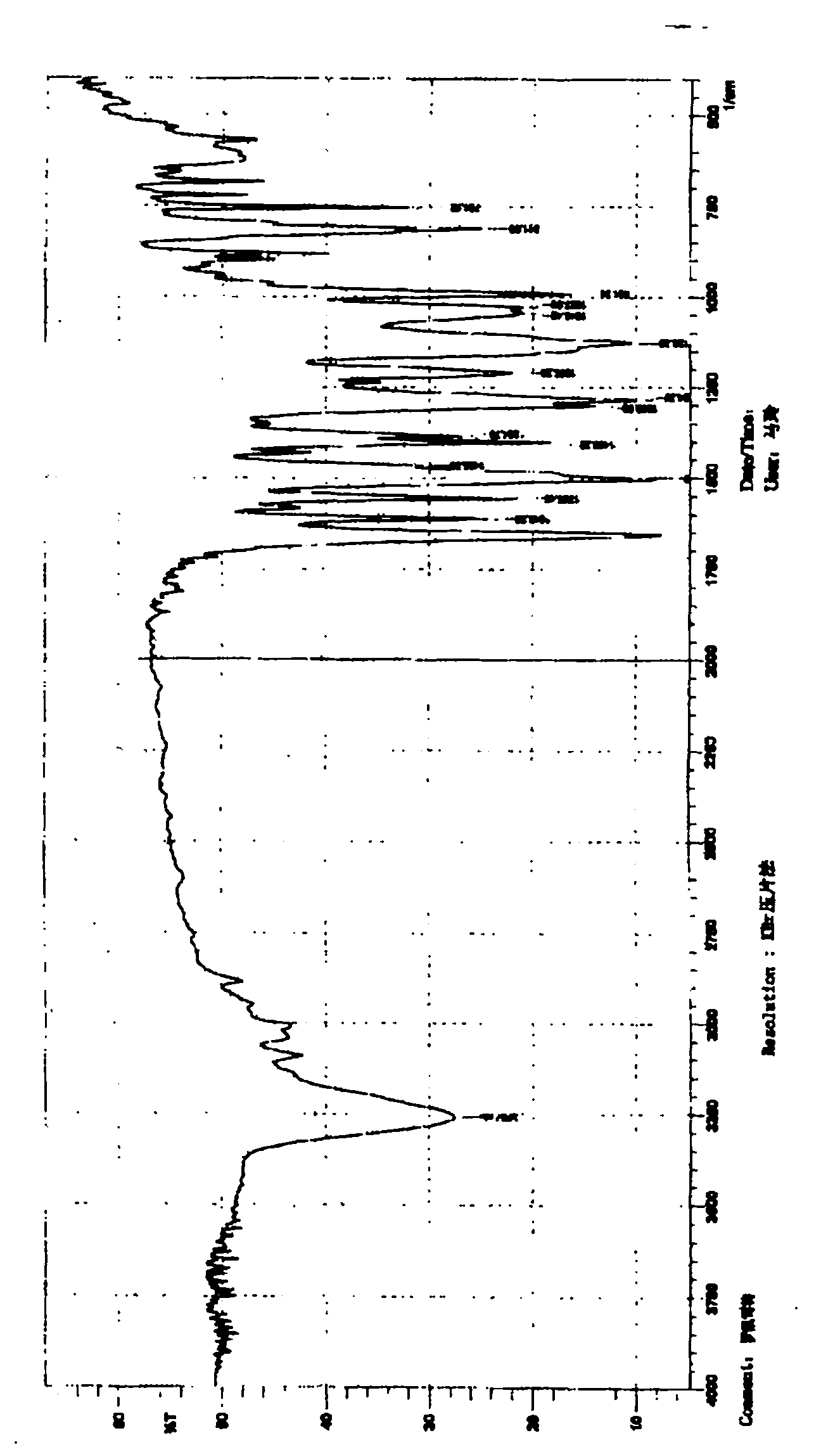
[0024] Suspend 5 grams of roflumilast crude product in 5 times the amount of ethyl acetate (w / v), heat to dissolve, add about 0.5 grams of activated carbon for decolorization, filter while hot, cool and crystallize, filter, and dry to constant weight. That is to say, the new crystalline state of roflumilast according to the present invention has a melting point of 161-162° C., the total amount of related substances detected by HPLC (normalization method) is less than 0.5%, and any largest single impurity does not exceed 0.1%.
Embodiment 2
[0026] Suspend 5 g of roflumilast crude product in 10 times the amount of anhydrous ether (w / v), heat to dissolve, add about 0.5 g of activated carbon for decolorization, filter while hot, freeze crystallization, filter, dry to constant weight, That is to say, the new crystalline state of roflumilast according to the present invention has a melting point of 162-163° C., and the total amount of related substances detected by HPLC (normalization method) is less than 0.4%, and any largest single impurity does not exceed 0.1%.
Embodiment 3
[0028] Suspend 5 grams of roflumilast crude product in 10 times the amount of acetone (w / v), heat to dissolve it, add about 0.5 grams of activated carbon for decolorization, filter while it is hot, cool and crystallize, filter, dry to constant weight, and obtain The new crystalline state of roflumilast according to the present invention has a melting point of 160-163° C., the total amount of related substances detected by HPLC (normalization method) is less than 0.4%, and any largest single impurity does not exceed 0.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com