Roflumilast solid dispersoid and preparation method thereof as well as roflumilast preparation
A technology of solid dispersion and roflumilast, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, active ingredients of heterocyclic compounds, etc., can solve the problems of low bioavailability and slow dissolution rate, and achieve bioavailability high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention provides a preparation method of roflumilast solid dispersion, comprising the following steps:
[0041] a), the carrier is heated and melted to obtain a carrier melt;
[0042] The carrier is copovidone, poloxamer or polyethylene glycol;
[0043] b), the carrier melt is mixed with Roflumilast;
[0044] c), mixing the obtained mixture, cooling and crushing to obtain a solid dispersion of roflumilast.
[0045] In the present invention, the carrier is first heated and melted to obtain a carrier melt. The carrier is copovidone, poloxamer or polyethylene glycol.
[0046] The carrier melt and roflumilast are then mixed. The mass ratio of the carrier to roflumilast is preferably 3-10:1. The mixing method of the carrier melt and roflumilast is preferably stirring.
[0047] In some embodiments of the present invention, the solubilizer is preferably added during the mixing process of the carrier melt and roflumilast, and the process is: mixing the carrier melt,...
Embodiment 1
[0072] Preparation of Roflumilast Solid Dispersion
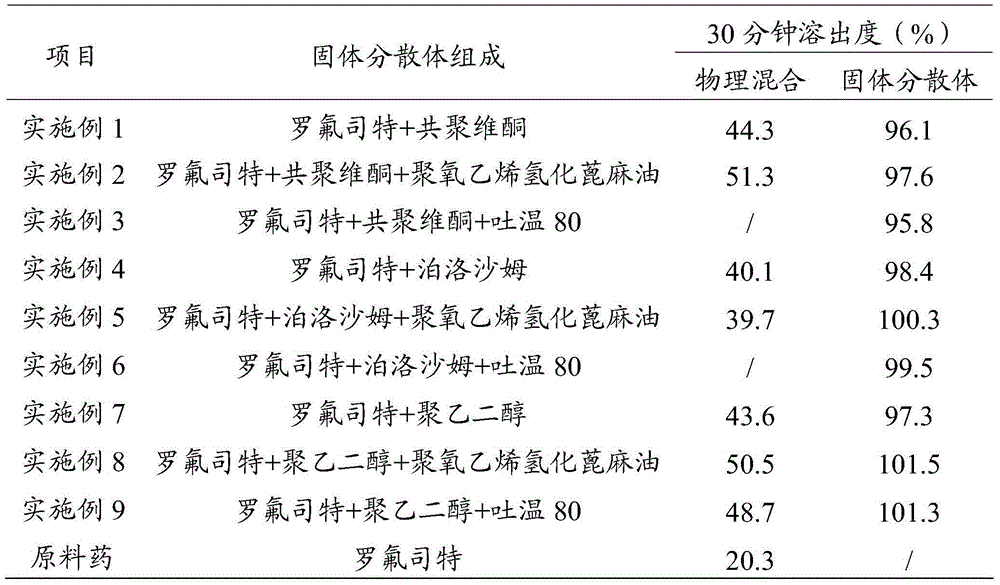
[0073] 5g copovidone (S-630) was heated and melted to obtain a melt. Add 1 g of roflumilast to the melt, stir to dissolve. The copovidone melt dissolved with roflumilast was poured on an iron plate at -20°C and cooled rapidly. The obtained solid was cooled and pulverized and passed through an 80-mesh sieve to obtain a solid dispersion of roflumilast.
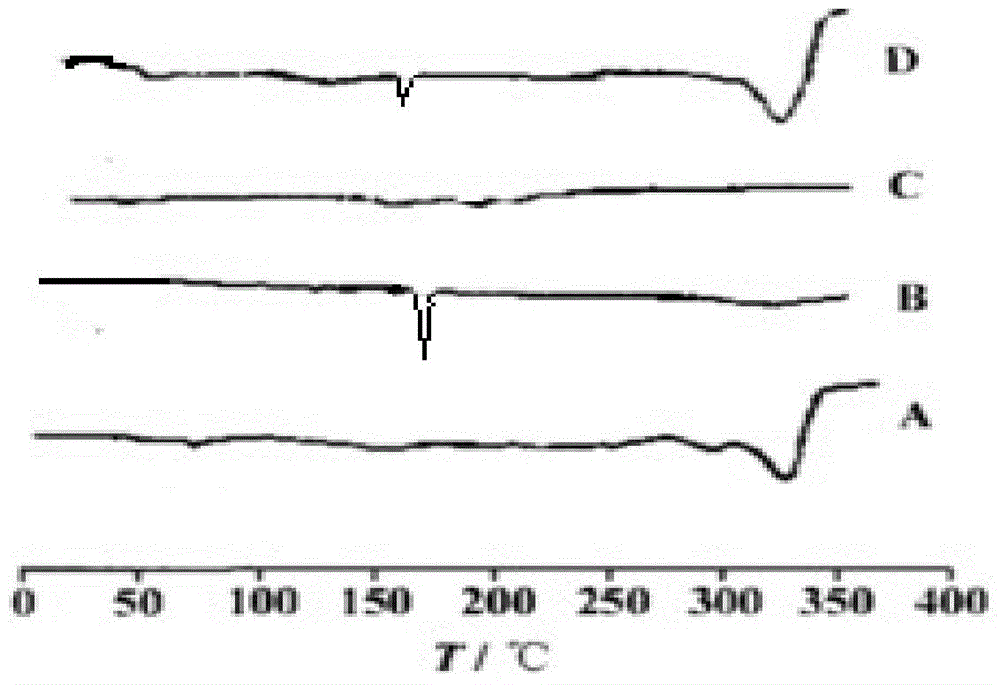
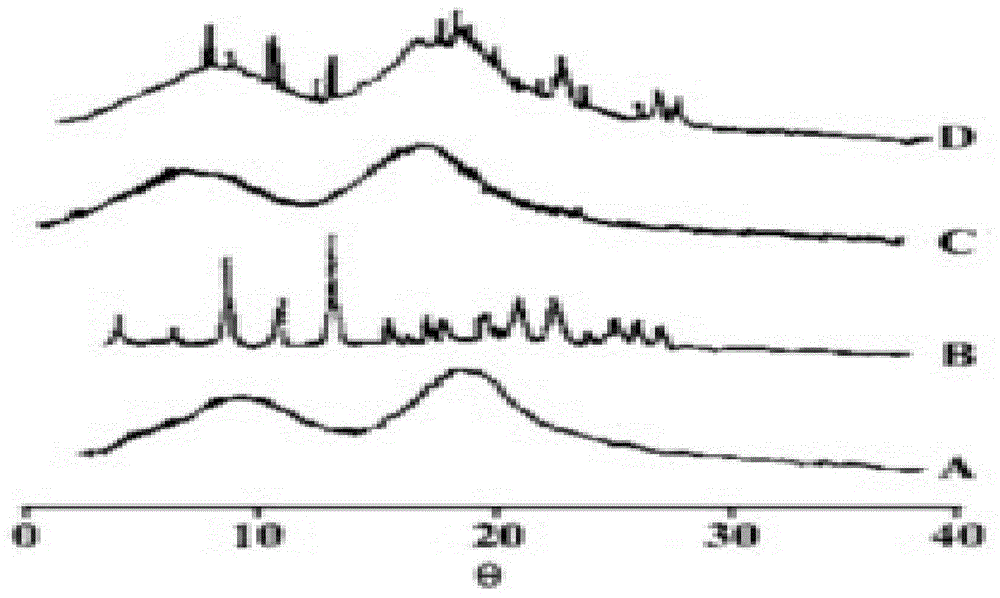
[0074] Carry out DSC scanning and X-ray powder diffraction analysis to the above-mentioned obtained roflumilast solid dispersion, get copovidone-roflumilast physical mixture, pure copovidone and pure roflumilast parallel determination simultaneously, the result Such as figure 1 and figure 2 as shown, figure 1 It is the DSC scanning figure of the roflumilast solid dispersion prepared in Example 1 of the present invention, wherein A is pure copovidone, B is pure roflumilast, C is roflumilast solid dispersion, and D is copolymerization Vitone-roflumilast physical mixture; ...
Embodiment 2
[0077] Preparation of Roflumilast Solid Dispersion
[0078] 5g copovidone (S-630) was heated and melted to obtain a melt. Add 100 mg of polyoxyethylene hydrogenated castor oil (RH-40) to the melt, stir to dissolve, then add 1 g of roflumilast, and stir to dissolve. The copovidone melt dissolved with polyoxyethylene hydrogenated castor oil and roflumilast was poured on an iron plate at -20°C, and cooled rapidly. The obtained solid was cooled and pulverized and passed through an 80-mesh sieve to obtain a solid dispersion of roflumilast.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com