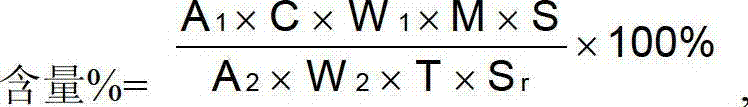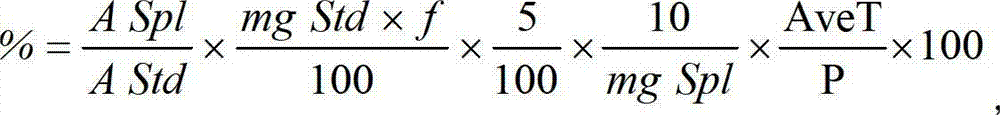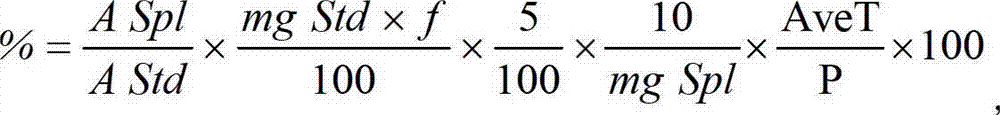Roflumilast tablets as well as preparation method and detection method thereof
A technology of roflumilast tablets and roflumilast, which is applied in the field of western medicine and can solve the problems of poor fluidity and compressibility, slow dissolution rate of starch, slow dissolution rate of tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0543] Embodiment 1: each tablet preparation is to use 0.5mg roflumilast as raw material drug, add 100mg lactose, 80mg pregelatinized starch, 10mg povidone K30 and 0.5mg magnesium stearate, and add acetone, ethanol, purified water made as a solvent. Pass lactose and pregelatinized starch through a 60-mesh sieve, and add them to a multi-functional coating granulator; stir roflumilast with acetone, ethanol, and purified water, and then add povidone K30 to make roflumilast Roflumilast solution, lactose, and pregelatinized starch were granulated using fluidized bed spray granulation equipment, dried, and granulated; adding magnesium stearate after passing through a 100-mesh sieve and mixing evenly; tableting, Roflumilast tablets are obtained after coating.
Embodiment 2
[0544] Embodiment 2: each tablet preparation is to use roflumilast 0.5mg as raw material drug, add 150mg lactose, 120mg pregelatinized starch, 30mg povidone K30 and 2.6mg magnesium stearate, and add acetone, ethanol, purified water made as a solvent. Pass lactose and pregelatinized starch through a 60-mesh sieve, and add them to a multi-functional coating granulator; stir roflumilast with acetone, ethanol, and purified water, and then add povidone K30 to make roflumilast Roflumilast solution, lactose, and pregelatinized starch were granulated using fluidized bed spray granulation equipment, dried, and granulated; adding magnesium stearate after passing through a 100-mesh sieve and mixing evenly; tableting, Roflumilast tablets are obtained after coating. Wherein, preparation process parameter is:
[0545] (1) Granulation process parameters: fan flow rate 35 ~ 120m 3 / h, peristaltic pump speed 6±2rpm, atomization pressure 0.2±0.02MPa, inlet air temperature 75±10℃, material te...
Embodiment 3
[0551] Embodiment 3: each tablet preparation is to use 0.5mg roflumilast as raw material drug, add 138.5mg lactose, 100mg pregelatinized starch, 20mg povidone K30, 1mg magnesium stearate, and add 0.02mL acetone, 0.2mL Ethanol, 0.13mL purified water as solvent. Pass lactose and pregelatinized starch through a 60-mesh sieve, and add them to a multi-functional coating granulator; stir roflumilast with acetone, ethanol, and purified water, and then add povidone K30 to make roflumilast Roflumilast solution, lactose, and pregelatinized starch were granulated using fluidized bed spray granulation equipment, dried, and granulated; adding magnesium stearate after passing through a 100-mesh sieve and mixing evenly; tableting, Serve immediately after coating. Wherein, the specific preparation method is as follows:
[0552] 1. Pretreatment of raw and auxiliary materials: pass lactose and pregelatinized starch through a 60-mesh sieve, weigh the above auxiliary materials in 3 equal parts,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com