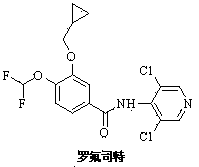
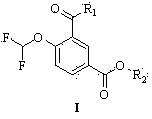
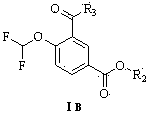
Method for preparing roflumilast intermediate
A technology of difluoromethylation and tetrabutylammonium fluoride, applied in the preparation of roflumilast, the intermediate of roflumilast and the field of preparation thereof, can solve the problem of high reagent cost and easy explosion of sodium nitrite , industrial production safety hazards and other issues, to achieve the effects of simple and easy availability of reagents, low reaction conditions and equipment requirements, and overcoming poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of methyl 3-formyl-4-difluoromethoxybenzoate
[0065] Dissolve 30.6g of phosphorus oxychloride and 36.5g of N,N-dimethylformamide (DMF) in 50mL of acetonitrile, stir at 0°C, add 15.2g of methyl 4-hydroxybenzoate, stir at 25°C, monitor the reaction by TLC Completely evaporate the solvent, add water, adjust the pH to 5-6 with aqueous sodium hydroxide solution, extract with ethyl acetate, dry over anhydrous magnesium sulfate, concentrate, and recrystallize with ethyl acetate to obtain 15.9 g of 3-formyl-4-hydroxy Methyl benzoate, yield 88.3%.
[0066] Dissolve 12.6g of methyl 3-formyl-4-hydroxybenzoate and 7.94g of benzyltriethylammonium chloride in 50mL of DMF, stir, add 35mL of 0.4g / mL sodium hydroxide aqueous solution, and introduce Freon gas, The completion of the reaction was monitored by TLC. Water was added, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated, and column chromatographed to obtain 12.3 g of methyl 3-formyl...
Embodiment 2
[0068] Preparation of methyl 3-formyl-4-difluoromethoxybenzoate
[0069] Dissolve 29.8g of thionyl chloride and 74.3g of N-methyl-N-phenylformamide in 100mL of tetrahydrofuran, stir at 2°C, add 15.2g of methyl 4-hydroxybenzoate, stir at 10°C, TLC monitors that the reaction is complete , evaporate the solvent, add water, adjust the pH to 5-6 with aqueous sodium carbonate solution, extract with ethyl acetate, dry over anhydrous magnesium sulfate, concentrate, and recrystallize with ethyl acetate to obtain 15.3g of 3-formyl-4-hydroxybenzoic acid Methyl ester, yield 85.0%.
[0070] Dissolve 12.6g of methyl 3-formyl-4-hydroxybenzoate and 5.17g of tetrabutylammonium iodide in 50mL of dioxane, stir, add 32.3g of diisopropylethylamine and 42.6g of difluorochloro Sodium acetate, continue to stir, TLC monitors that the reaction is complete, evaporate the solvent, add water, extract with ethyl acetate, dry over anhydrous sodium sulfate, concentrate, and column chromatography to obtain...
Embodiment 3
[0072] Preparation of methyl 3-formyl-4-difluoromethoxybenzoate
[0073] Dissolve 15.2g of methyl 4-hydroxybenzoate and 1.53g of tetramethylammonium bromide in 100mL of acetonitrile, add 25.4mL of 2.5g / mL sodium carbonate aqueous solution and 72.0g of methyl difluorochloroacetate, stir, and monitor the reaction by TLC Completely evaporate acetonitrile, add water, extract with dichloromethane, dry over anhydrous sodium sulfate, concentrate, and perform column chromatography to obtain 16.5 g of methyl 4-difluoromethoxybenzoate with a yield of 81.7%.
[0074] Dissolve 26.6g of magnesium chloride and 48.3g of N-formylmorpholine in 50mL of dimethyl sulfoxide, stir at 0°C, add 14.1g of methyl 4-difluoromethoxybenzoate, stir at 20°C, TLC monitors the reaction is complete, Evaporate the solvent, add water, adjust the pH to 5-6 with an aqueous potassium carbonate solution, extract with dichloromethane, dry over anhydrous magnesium sulfate, and recrystallize from ethyl acetate to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com