Roflumilast tablet and preparation method thereof
A technology of roflumilast tablets and roflumilast, which is applied in the directions of pharmaceutical formulations, medical preparations of inactive ingredients, and pill delivery, etc., can solve the problems of undisclosed, slow drug speed, and slow tablet disintegration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035]
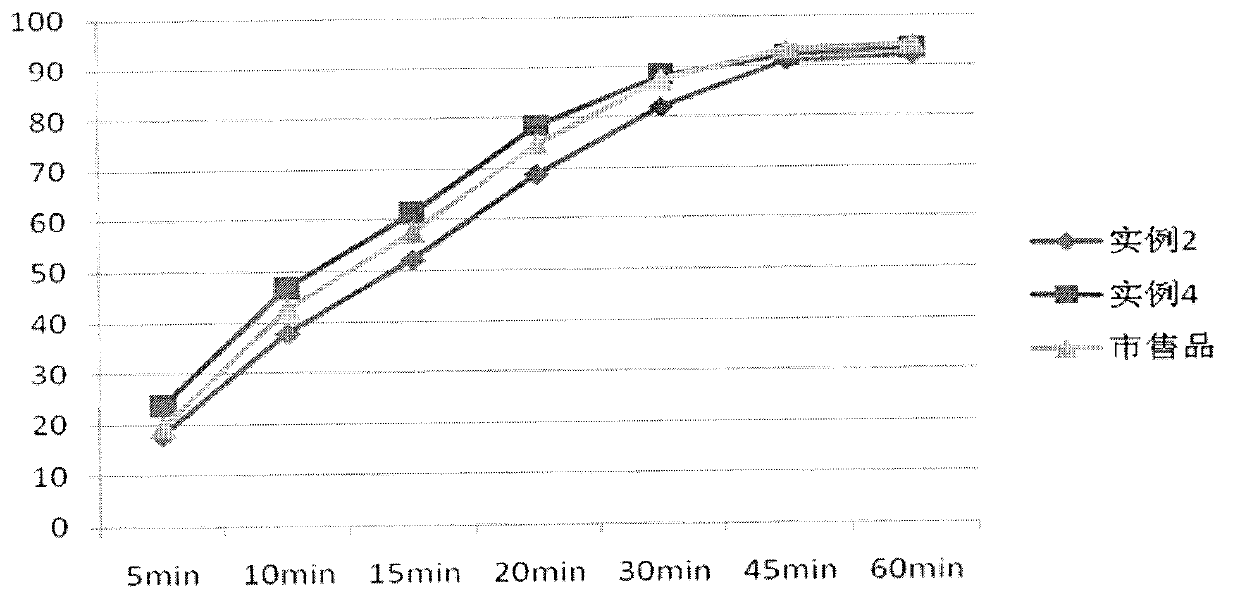
[0036] Preparation method: pulverize the raw material of Roflumilast with a jet mill, weigh out 1000 tablets of the raw material of Roflumilast, and use them for later use; use the method of adding the same amount of the raw material of Roflumilast to the prescription amount. The croscarmellose sodium is thoroughly mixed, and then it is thoroughly mixed with the prescribed amount of microcrystalline cellulose, direct-pressed lactose, and magnesium stearate; the bulk density and angle of repose of the mixed powder are measured, and the pressure is converted according to the content For tablet weight, press the tablet to adjust the hardness of the tablet to 2~3kg, with a difference of ±5.0%.
Embodiment 2
[0038]
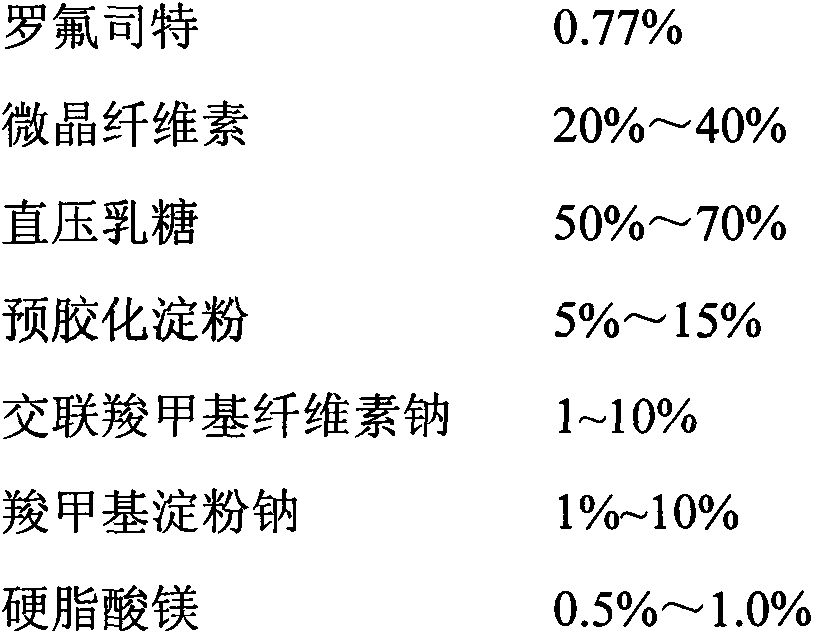
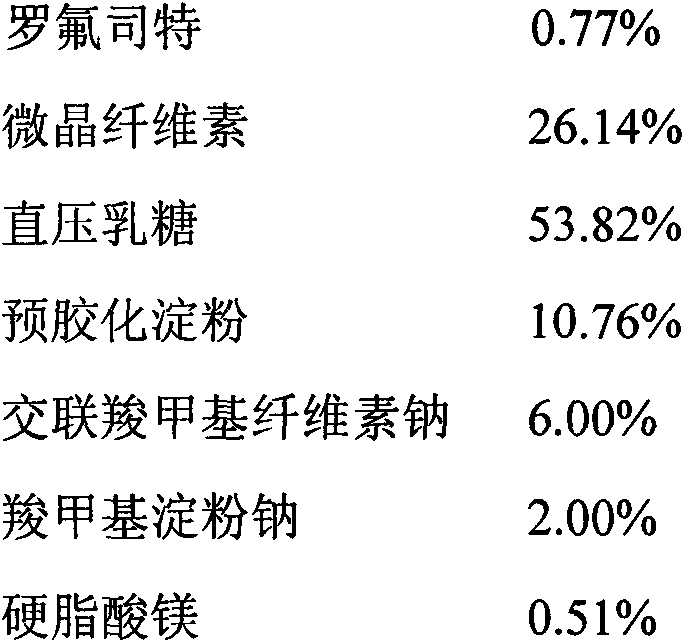
[0039] Preparation method: pulverize the raw material of Roflumilast with a jet mill, weigh out 1000 tablets of the raw material of Roflumilast, and reserve them for use; adopt the method of adding the same amount, first combine the raw material of Roflumilast with the prescription amount The croscarmellose sodium is thoroughly mixed, and then it is thoroughly mixed with the prescribed amount of pregelatinized starch, microcrystalline cellulose, direct-pressed lactose, and magnesium stearate; after the content of the mixed material is tested, the content is converted according to the content The tablet should be compressed, and the hardness of the tablet should be adjusted to 2~3kg, with a difference of ±5.0%.
Embodiment 3
[0041]
[0042]
[0043] Preparation method: pulverize the raw material of Roflumilast with a jet mill, weigh out 1000 tablets of the raw material of Roflumilast, and use them for later use; use the method of adding the same amount of the raw material of Roflumilast to the prescription amount. Sodium carboxymethyl starch is thoroughly mixed, and then it is thoroughly mixed with the prescribed amount of pregelatinized starch, microcrystalline cellulose, straight-pressed lactose, and magnesium stearate; after the mixed powder is tested for the angle of repose and bulk density content, according to The content should be converted into the weight of the tablet, and the tablet should be compressed to adjust the hardness of the tablet to 2~3kg, and the difference in the filling amount is ±5.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com