Tablet containing roflumilast as active ingredients and preparation method of tablet
An active ingredient, a technology of roflumilast, which is applied to the field of tablets containing roflumilast as an active ingredient and the preparation thereof, can solve the problems of complex process, slow dissolution rate and high operation cost, and achieves simple process and cost saving Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
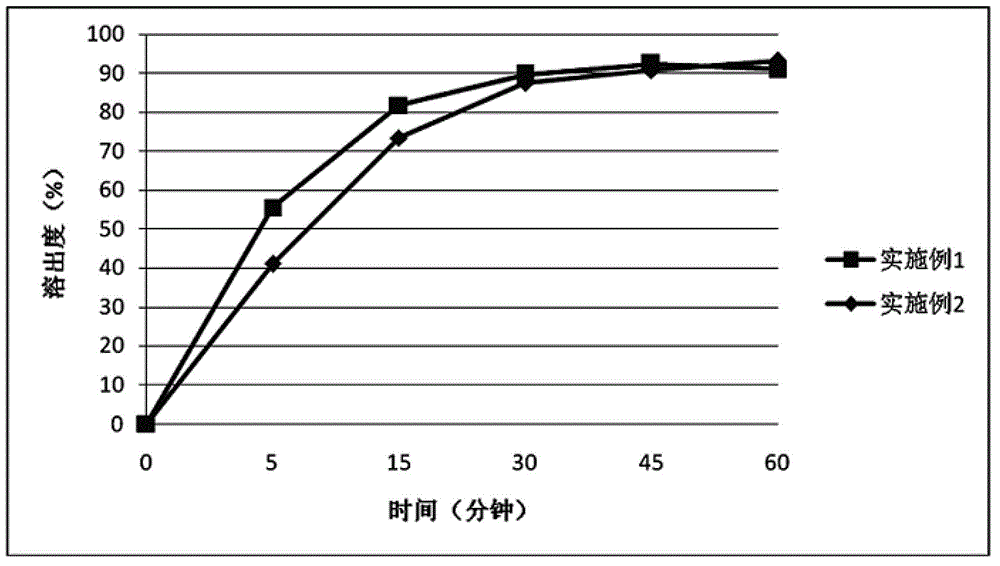
Embodiment 1
[0022] The tablet containing roflumilast as an active ingredient described in the embodiments of the present invention contains:
[0023] A: Roflumilast (through a 150-mesh sieve) 0.5 mg;
[0024] B: Lactose monohydrate 99.32 mg;
[0025] C: Corn starch 26.78 mg;
[0026] D: Hydroxypropyl methylcellulose (viscosity 4~6mP·s) 1 mg;
[0027] E: water 3 mg;
[0028] F: Ethanol 22 mg;
[0029] End result: 128.2 mg / tablet.
[0030] The preparation method of the tablet containing roflumilast as the active ingredient described in the embodiment of the present invention comprises the following steps:
[0031] (1) Mix all pharmaceutical excipients except hydroxypropyl methylcellulose;
[0032] (2) Dissolve or suspend hydroxypropyl methylcellulose and roflumilast respectively in the mixed solution of ethanol and water;
[0033] (3) The mixture obtained in step (1) and the solution obtained in (2) were granulated in a wet granulator, and the wet granules were dried and sized befo...
Embodiment 2
[0035] The tablet containing roflumilast as an active ingredient described in the embodiments of the present invention contains:
[0036] A: Roflumilast (through a 150-mesh sieve) 0.5 mg;
[0037] B: Lactose monohydrate 99.32 mg;
[0038] C: Corn starch 26.78 mg;
[0039] D: Hydroxypropyl methylcellulose (viscosity 4~6mP·s) 1 mg;
[0040] E: Water 3.6 mg;
[0041] F: Ethanol 18 mg;
[0042] End result: 128.2 mg / tablet.
[0043] The preparation method of the tablet containing roflumilast as the active ingredient described in the embodiment of the present invention comprises the following steps:
[0044] (1) Mix all pharmaceutical excipients except hydroxypropyl methylcellulose;
[0045] (2) Dissolve or suspend hydroxypropyl methylcellulose and roflumilast respectively in the mixed solution of ethanol and water;
[0046] (3) The mixture obtained in step (1) and the solution obtained in step (2) were granulated in a wet granulator, and the wet granules were dried and siz...
Embodiment 3
[0048] The tablet containing roflumilast as an active ingredient described in the embodiments of the present invention contains:
[0049] A: Roflumilast (through a 150-mesh sieve) 0.5 mg;
[0050] B: Microcrystalline cellulose 105.32 mg;
[0051] C: Pregelatinized starch 20.18 mg;
[0052] D: Hydroxypropyl methylcellulose (viscosity 40~60mP·s) 0.5 mg;
[0053] E: water 2 mg;
[0054] F: 30 mg ethanol;
[0055] End result: 126.5 mg / tablet.
[0056]The preparation method of the tablet containing roflumilast as the active ingredient described in the embodiment of the present invention comprises the following steps:
[0057] (1) Mix all pharmaceutical excipients except hydroxypropyl methylcellulose;
[0058] (2) Dissolve or suspend hydroxypropyl methylcellulose and roflumilast respectively in the mixed solution of ethanol and water;
[0059] (3) The mixture obtained in step (1) and the solution obtained in step (2) are granulated in a fluidized bed granulator, and the wet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com