Method for reducing side effects from administration of phosphodiesterase-4 inhibitors
a phosphodiesterase and inhibitor technology, applied in the field of topical medications, can solve the problems of high incidence of diarrhea, nausea and abdominal pain, sharp spike in plasma concentration, etc., and achieve the effect of reducing gastrointestinal side effects, reducing cmax spike, and producing high au
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ons According to the Invention and of the Prior Art
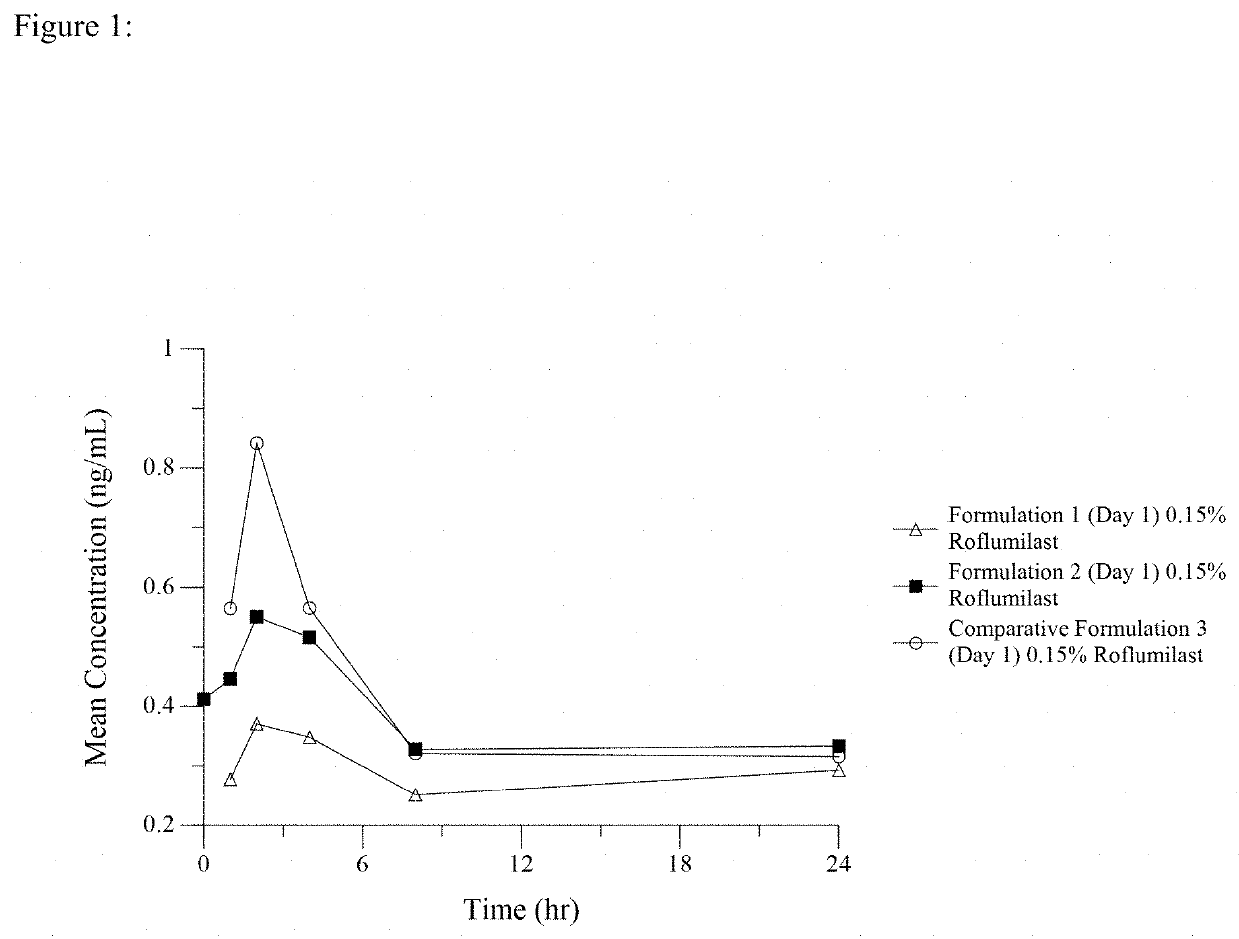
[0072]A first formulation of the invention, hereafter referred to as Formulation 1, was made by combining roflumilast with a phosphate ester surfactant and water. The formulation was buffered with NaOH to obtain a pH of 6.5.
[0073]A second formulation of the invention, hereafter referred to as Formulation 2, was made by combining the above constituents and adding diethylene glycol monoethyl ether. This formulation was buffered with NaOH to obtain a pH of 6.5.
[0074]A formulation that is not of the invention, hereafter referred to as Comparative Formulation 3, was made by combining roflumilast with diethylene glycol monoethyl ether. This formulation was gelled with hydroxylpropyl cellulose so that it would have a similar viscosity and spread on the skin like the two phosphate ester surfactant emulsion Formulations 1, and 2. This semisolid formulation was likewise buffered with NaOH to obtain a pH of 6.5.
[0075]The compositions of these ...
example 2
se Testing of Formulations of Example 1
[0076]Male and female swine (Gottingen Minipig® breed) (Marshall BioResources, North Rose, N.Y.) were ordered to weigh 8 to 12 kg at arrival. On the day prior to administration of one of the topical cream semisolid formulations of Example 1 containing 0.15% roflumilast, the hair was clipped from the back of each animal. The pigs were sedated for the shaving procedure. Care was taken to avoid abrading the skin.
[0077]Two (2) grams of one of the cream formulations of Example 1 for each kg of pig weight was distributed over the clipped skin area by gentle inunction with a glass stirring rod or stainless-steel spatula. The cream formulation was applied evenly with a thin, uniform film beginning at the scapular region and moving caudally over the test site. The width of the test site area was bilaterally divided by the spine. Six pigs (3 males and 3 females) were administered a single dose of the Formulation 2. Blood was sampled from the anterior ven...
example 3
on of the Invention and a Formulation of the Closest Prior Art
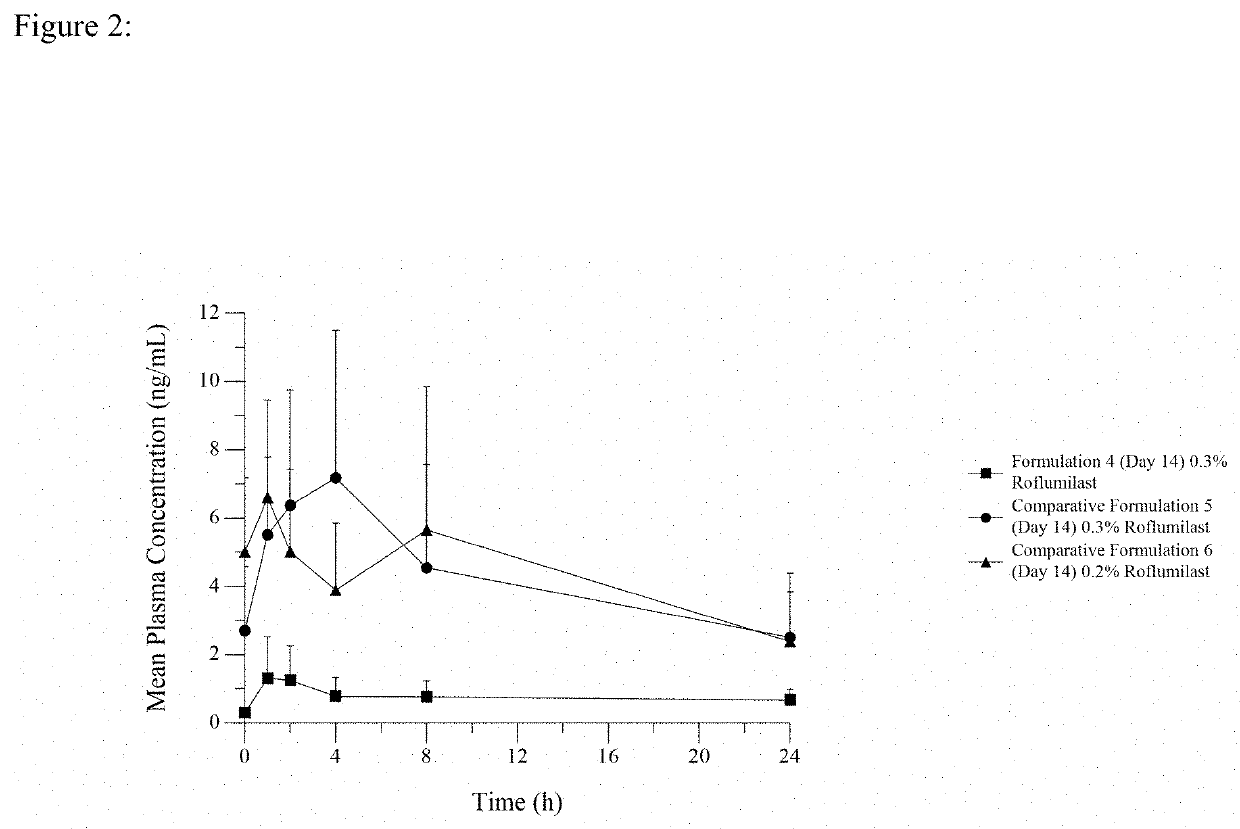
[0080]A third embodiment of the invention, hereafter referred to as Formulation 4, was made by combining roflumilast at a concentration of 0.3% w / w with a phosphate ester surfactant and water. The formulation was buffered with NaOH to obtain a pH of 5.5. This formulation is similar to Formulation 1 except that the concentration of roflumilast is 0.3% rather than 0.15% and the emulsion is buffered to a pH value of 5.5 rather than a pH value of 6.5.
[0081]A formulation that is not of the invention, hereafter referred to as Comparative Formulation 5, was made by combining roflumilast at a concentration of 0.3% containing a phosphate ester surfactant, a polyoxyl stearyl ether surfactant and diethylene glycol monoethyl ether, as well as other excipients. This formulation is a cream formulation containing a frequently used phosphate ester surfactant that is not Crodafos CES.
[0082]A formulation that is not of the invention, herea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com