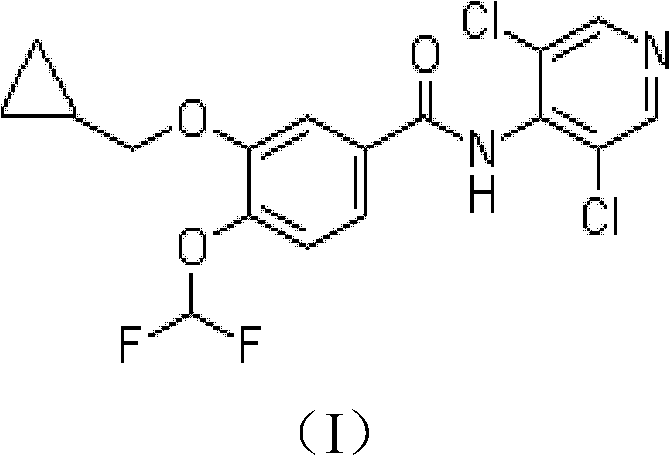
Method for preparing high-purity roflumilast
A compound, selected technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of expensive, complicated operation, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
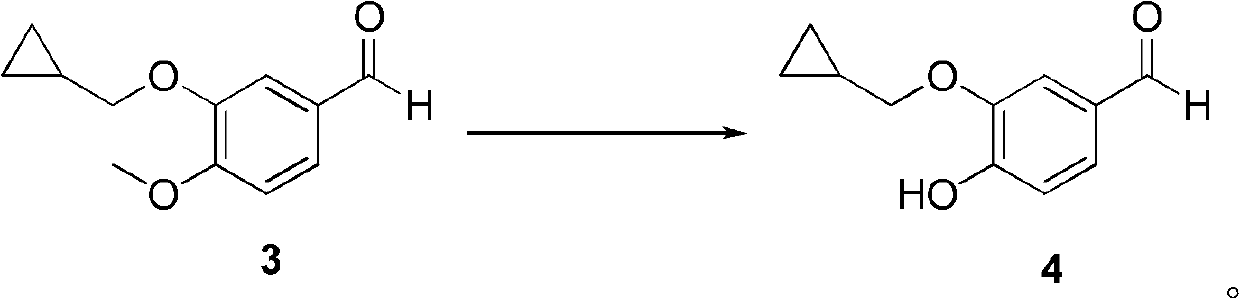
[0054] Embodiment 1: Preparation of 3-(cyclopropylmethoxy)-4-methoxybenzaldehyde (compound 3)
[0055] Add 3-hydroxy-4-methoxybenzaldehyde (compound 1, 15.2g, 0.1mol), bromomethylcyclopropane (compound 2, wherein X is Br, 16.2g, 0.12mol), potassium carbonate to the reaction flask (41.4 g, 0.36 mol) and DMF (200 mL). The mixture was reacted at room temperature for 8 hours, and the reaction was complete by TLC. Ethyl acetate (200 mL) was added to the reaction solution, followed by washing with distilled water. The aqueous and organic phases were separated, and the aqueous phase was extracted with ethyl acetate. The organic phases were combined and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain compound 3 as 20.2 g of off-white solid with a yield of 98%.
[0056] 1 H NMR (CDCl 3 ): δ9.84(1H, s), 7.47-7.44(1H, d, J=2.0Hz), 7.39(1H, s), 6.98-7.00(1H, d, J=8.4Hz), 3.97(3H, s), 3.97-3.92 (2H, d, J=22Hz), 1.30-1.32 (1H, m), 0...
Embodiment 2
[0057] Embodiment 2: Preparation of 3-(cyclopropylmethoxy)-4-hydroxybenzaldehyde (compound 4)
[0058] Compound 3 (10.3 g, 50 mmol) was dissolved in NMP (150 mL), and an aqueous solution of sodium thiophenate (~36.7% w / w, 0.1 mol) was added. The obtained mixed solution was stirred and reacted at 190-200° C. for 1 hour, and then cooled to room temperature. The reaction mixture was poured into distilled water (500 mL), extracted with ethyl acetate (2 x 100 mL). The aqueous phase was adjusted to pH 1-2 with hydrochloric acid (6.0M), and extracted with ethyl acetate (3×100 mL). The organic phases were combined and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain compound 4 as 7.68 g of off-white solid with a yield of 80%.
[0059] 1 H NMR (CDCl 3 ): δ9.81(1H, s), 7.43-7.40(1H, d, J=2.0Hz), 7.38(1H, s), 7.06-7.04(1H, d, J=4.0Hz), 3.96-3.94( 2H, d, J = 6.8 Hz), 1.33-1.29 (1H, m), 0.70-0.66 (2H, m), 0.39-0.36 (2H, m).
Embodiment 3
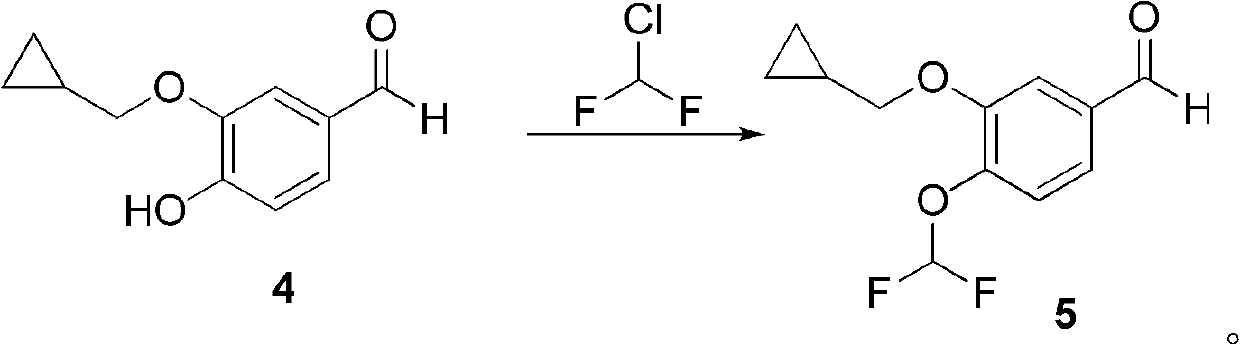
[0060] Embodiment 3: Preparation of 3-(cyclopropylmethoxy)-4-difluoromethoxybenzaldehyde (compound 5)
[0061] Compound 4 (5.77g, 30mmol), K 2 CO 3 (12.42g, 90mmol) and DMF (100mL) were mixed in a three-necked flask, and then Freon gas was introduced to react at 45°C for 6 hours. TLC showed the reaction was complete. The reaction solution was poured into distilled water, and extracted twice with ethyl acetate. The organic phase was washed with saturated sodium chloride and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain compound 5 as 7.05 g of yellow oily liquid with a yield of 97%.
[0062] 1 H NMR (CDCl 3 ): δ9.92(1H, s), 7.47-7.44(1H, d, J=2.0Hz), 7.43(1H, s), 7.32-7.30(1H, d, J=4.0Hz), 6.94-6.57( 1H, d, J = 150Hz), 3.96-3.94 (2H, d, J = 6.8Hz), 1.34-1.30 (1H, m), 0.70-0.65 (2H, m), 0.39-0.36 (2H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com