Method for synthesizing (trans)-4-alkyl-3-alkene biphenyl derivative monomer liquid crystals
A synthesis method and derivative technology, applied in liquid crystal materials, chemical instruments and methods, hydrocarbons, etc., can solve problems such as cost increase, catalyst cannot be recycled and applied, limited industrial use, etc. The effect of mild production and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
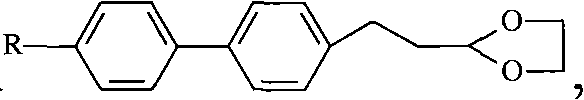
[0039] Step 1, the preparation of 2-[2-(4-bromophenyl)ethene]-[1,3]dioxane
[0040] Add 75g of chemical formula II (p-bromocinnamaldehyde), 31g of ethylene glycol, 500ml of toluene, and 1g of p-toluenesulfonic acid into a 1L three-necked flask. Water, when no more water comes out after one hour, cool down to room temperature with a cold water bath, wash the reaction solution with water until the pH value of the washing water is neutral, dry with anhydrous sodium sulfate for 5 hours, distill off toluene under reduced pressure, and the residual The substance is heated and dissolved with 0.5 times of toluene and 1 times of petroleum ether, then frozen, filtered to obtain 75g of white crystal 2-[2-(4-bromobenzene)ethylene]-[1,3]dioxane, yield 84% .
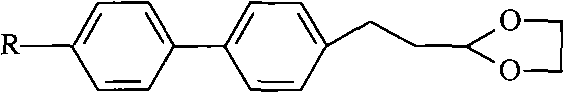
[0041] Step 2, the preparation of 2-[2-(4'-methyl-biphenyl-4-)-ethylene]-[1,3-dioxane]
[0042] Add 27.2g of p-tolueneboronic acid, 51g of 2-[2-(4-bromobenzene)ethylene]-[1,3]dioxane, 25.5g of sodium carbonate, and 200ml of dimethyl...
Embodiment 2
[0054] The raw material that this embodiment uses is chemistry III (p-bromophenylpropanal), and its reaction steps are:
[0055] Step 1, Preparation of 2-[2-(4-bromophenyl)ethyl]-[1,3]dioxane
[0056] Using Chemistry III (p-bromophenylpropionaldehyde) as raw material, adopting the same reaction conditions as step one of Example 1, directly prepare 2-[2-(4-bromophenyl) ethyl]-[1,3] di Oxygen Pentacycline.
[0057] Step 2, the preparation of 2-[2-(4'-methyl-biphenyl-4-)-ethyl]-[1,3-dioxopentacycline]
[0058] The 2-[2-(4-bromophenyl) ethyl]-[1,3]dioxane prepared in step 1 of this example is used as raw material, and the reaction conditions are the same as step 2 in Example 1 to prepare 2 -[2-(4'-Methyl-biphenyl-4-)-ethyl]-[1,3-dioxolane].
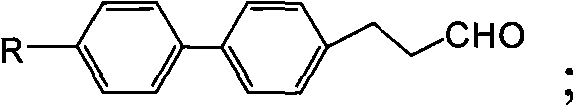
[0059] Step 3, the preparation of 3-(4'-methylbiphenyl-4-)propionaldehyde
[0060] Same as Step 4 of Example 1.
[0061] Step 4. Preparation of (cis, trans)-4'-methyl-4-(pent-3-enyl)biphenyl
[0062] Same as Step 5 of Example 1.
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com