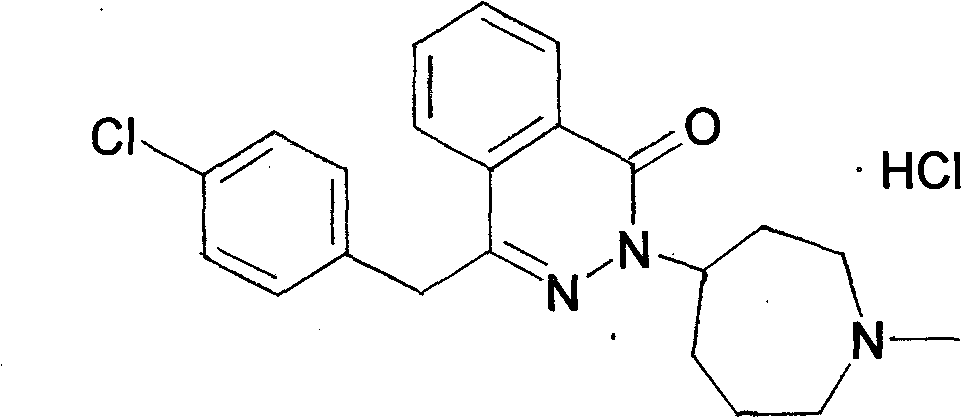
Method for synthesizing N-methylhexahydroazepin-4-one hydrochloride, azelastine hydrochloride intermediate
A technique for the synthesis of methylhexahydrogen, applied in the direction of organic chemistry, can solve the problems of high market price of ethyl ester, unfavorable industrial production, low yield of cyclization, etc., and achieve small amount of waste water, easy treatment, and process operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
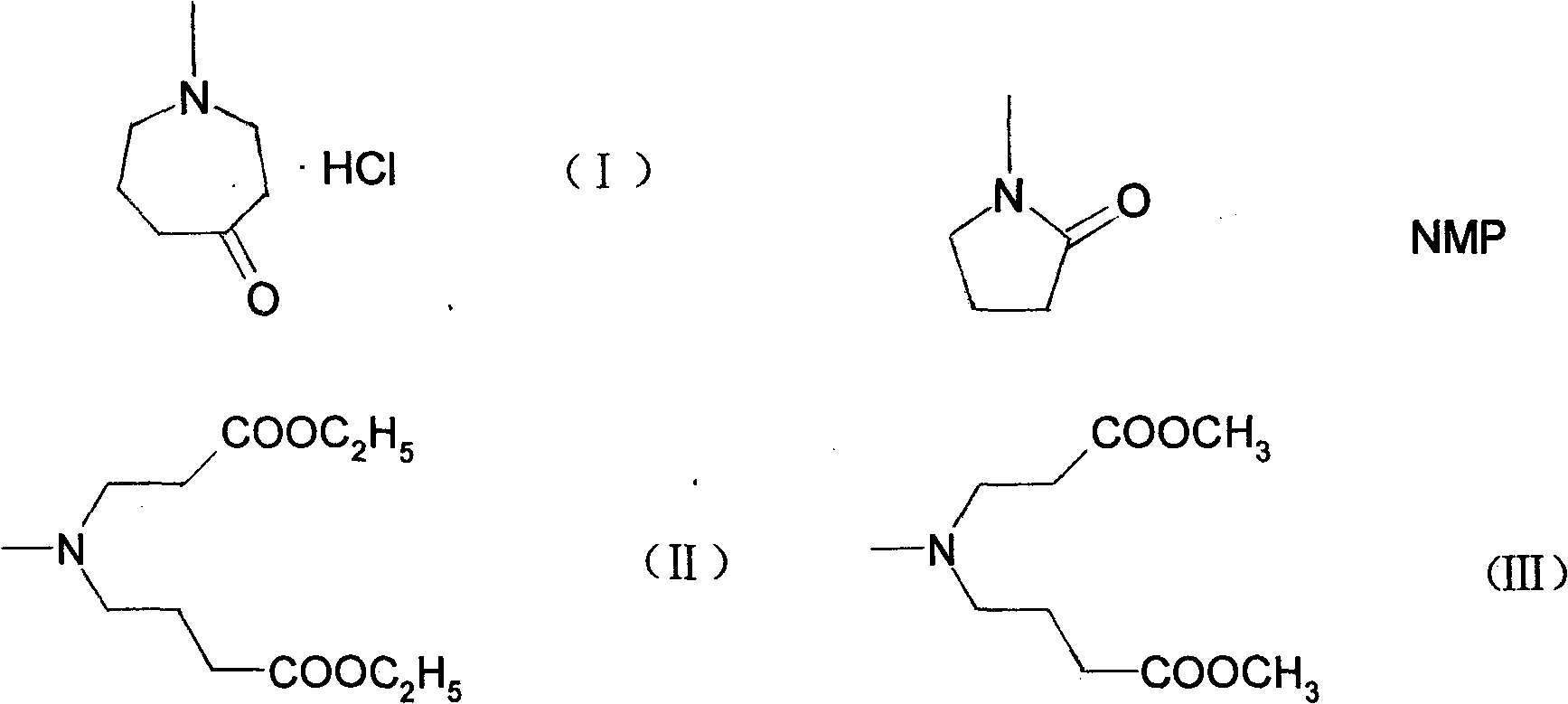
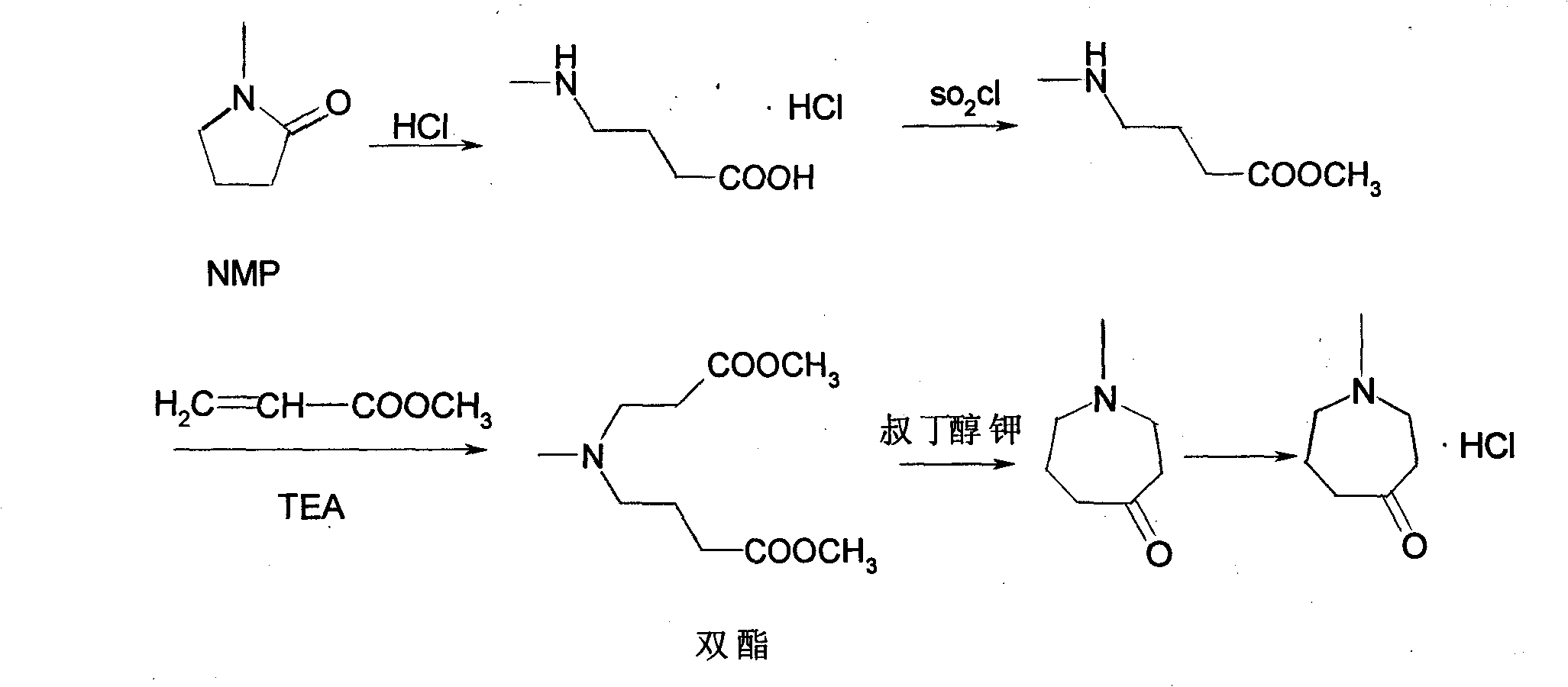
[0045] Embodiment 1: synthetic reaction route is as follows:
[0046]
[0047] (1) Add 18g of N-methyl-2-pyrrolidone to 37ml of concentrated hydrochloric acid and stir to reflux for 5 hours. Cool, evaporate the hydrochloric acid to dryness under reduced pressure, add 50ml of cold acetone to the remaining solid, stir, cool, the solid becomes crystallized, freeze to make it completely precipitate, filter, wash twice with 15ml of acetone, drain and dry, Obtained 24.2g, the total yield was 88.2%.
[0048] (2) Add 40ml of thionyl chloride dropwise to 150ml of anhydrous methanol under stirring at -5°C to 0°C in the external bath, keep the internal temperature not exceeding -3°C, and dropwise add in about 3 hours, then add step (1 ) product 20g, the reaction solution was reacted at 25°C for 12 hours, after the methanol was concentrated under reduced pressure, the mother liquor was stirred evenly for the next step.
[0049] (3) 22g of methyl acrylate, 20g of triethylamine and 150ml...
Embodiment 2
[0054] As described in Example 1, the difference is that the extractant in step (5) is chloroform to obtain 7.1 g of N-methylhexahydro-4-one hydrochloride with a yield of 94.3%.
Embodiment 3
[0056] As described in Example 1, the difference is that the extractant in step (5) is dichloroethane to obtain 6.8 g of N-methylhexahydro-4-one hydrochloride with a yield of 90.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com