Carbazole fluorescent probe for detecting ClO- and preparation method and application thereof
A fluorescent probe and carbazole-based technology, applied in the field of carbazole-based fluorescent probes and their preparation, can solve the problems of complex preparation methods, expensive raw materials, etc., and achieve simple reaction conditions, good cell membrane penetration, and sensitive reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 measures ClO - Preparation of Fluorescent Probes
[0033] (1) Synthesis of 9-butylcarbazole
[0034] Add 8.35g (0.050mol) of carbazole and 60mL of dimethyl sulfoxide into a 100mL three-necked flask equipped with a magnetic stirring device, a thermometer and a reflux condenser. After stirring to dissolve, quickly add 3.0g (0.054mol) Potassium hydroxide, heated to 90°C, reacted under stirring until the solids were completely dissolved, continued to react for 30 minutes, cooled, installed a constant pressure dropping funnel, and slowly added 5.5mL (0.051mol) bromobutyl dropwise after the reaction solution cooled to below 38°C alkyl. Keep stirring at 90°C for 4-6 hours. After the reaction is completed, pour it into ice water twice the volume while it is still hot, and let it stand overnight. A white precipitate precipitates out. Suction filtration, wash the filter cake with water 2-3 times, remove inorganic matter and dimethyl sulfoxide, and dry to obtain th...
Embodiment 2
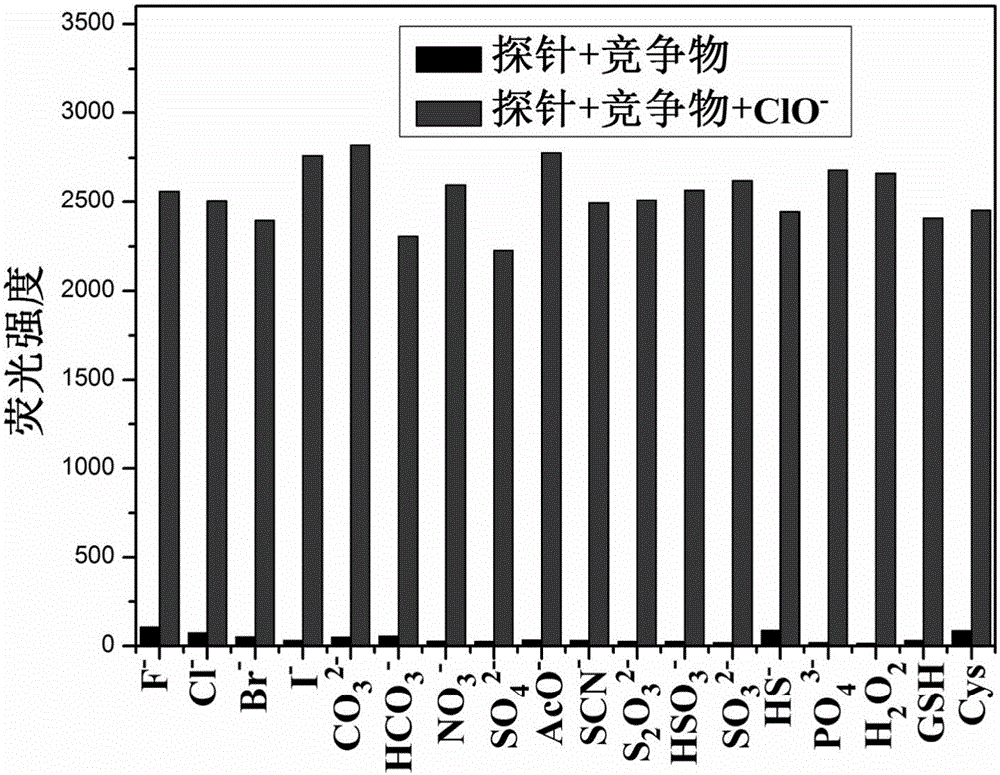
[0044] The ClO of embodiment 2 fluorescent probes - Fluorescence titration
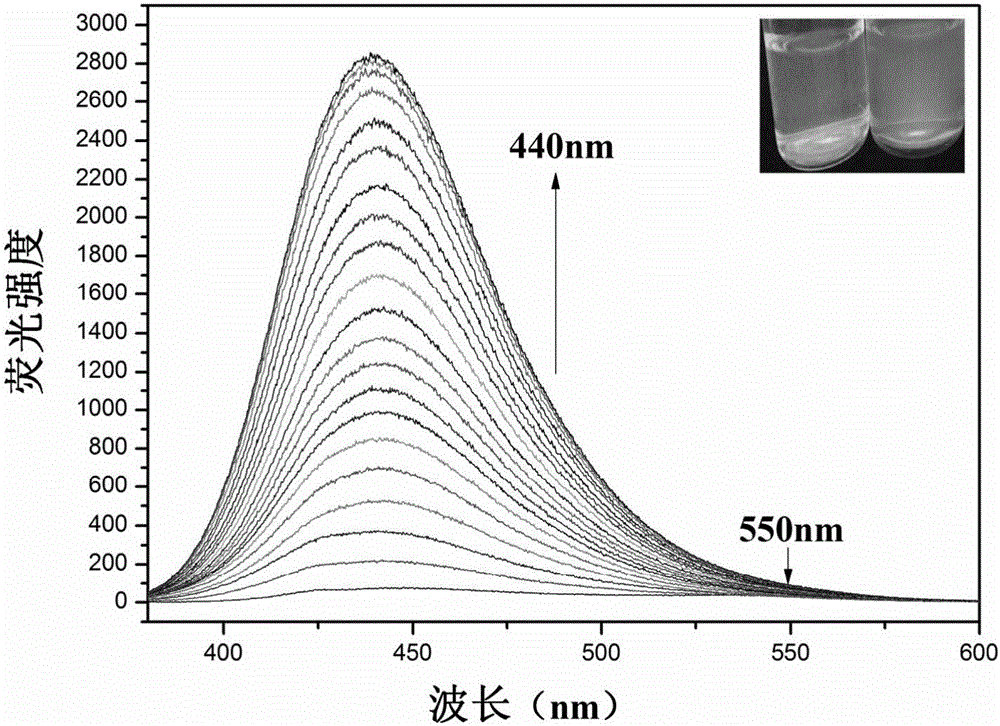
[0045] Add 20 μL fluorescent probe stock solution to 1.6 mL water and 0.4 mL ethanol system for ClO - In the fluorescence titration experiment, the fluorescence intensity at 440nm is gradually enhanced with the addition of the sample to be tested on a fluorescence spectrophotometer. Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 2.5nm and 5.0nm respectively, the voltage is 600V, and the maximum excitation wavelength of the fluorescent probe solution is: λ ex 370nm (see figure 1 ).
Embodiment 3
[0046] The ClO of embodiment 3 fluorescent probes - UV titration
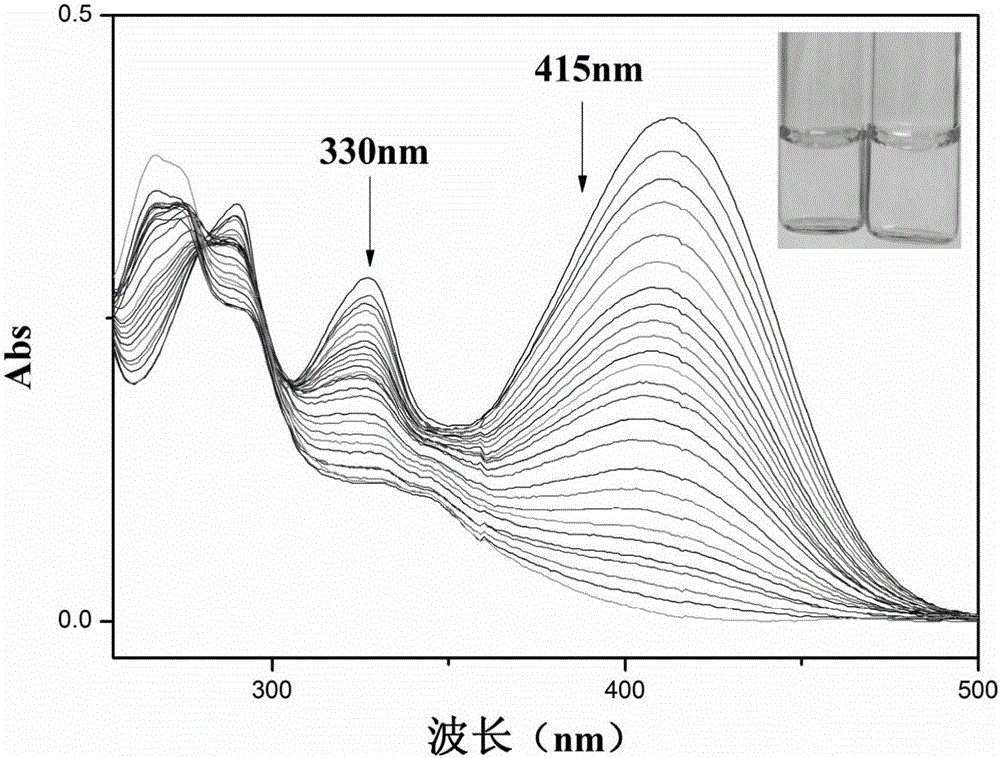
[0047] Add 30 μL fluorescent probe stock solution to 1.6 mL water and 0.4 mL ethanol system for ClO - The ultraviolet titration experiment is detected on the ultraviolet spectrophotometer. With the addition of the sample to be tested, the ultraviolet absorption intensity at 415nm is gradually weakened (see figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com