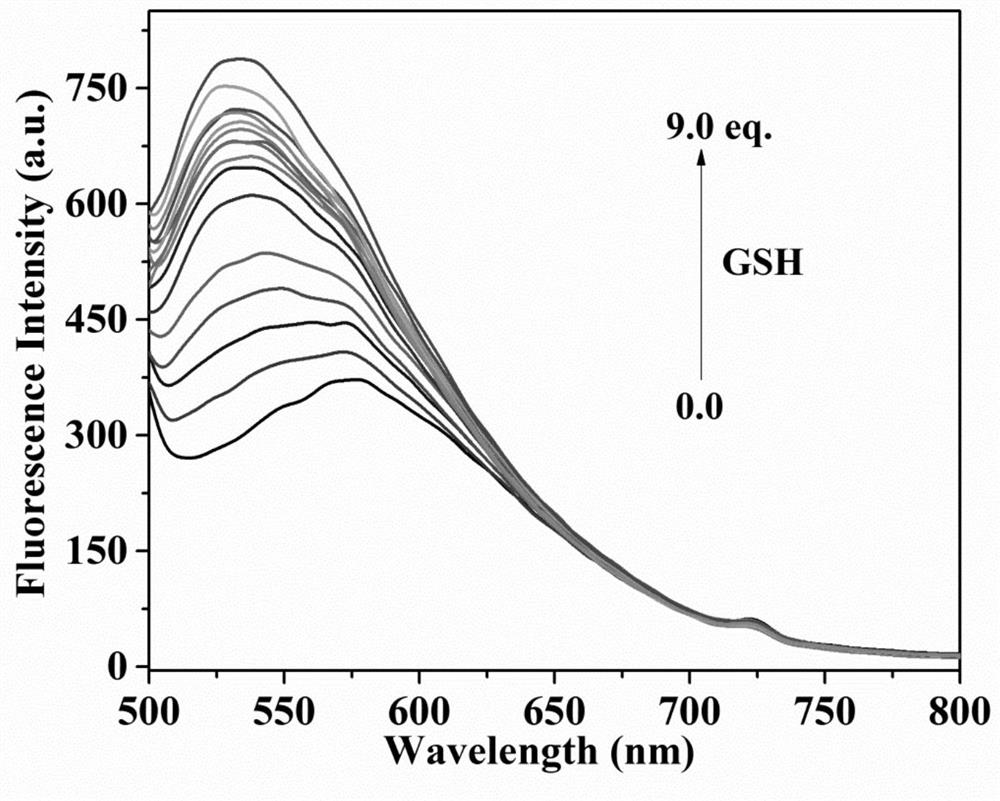
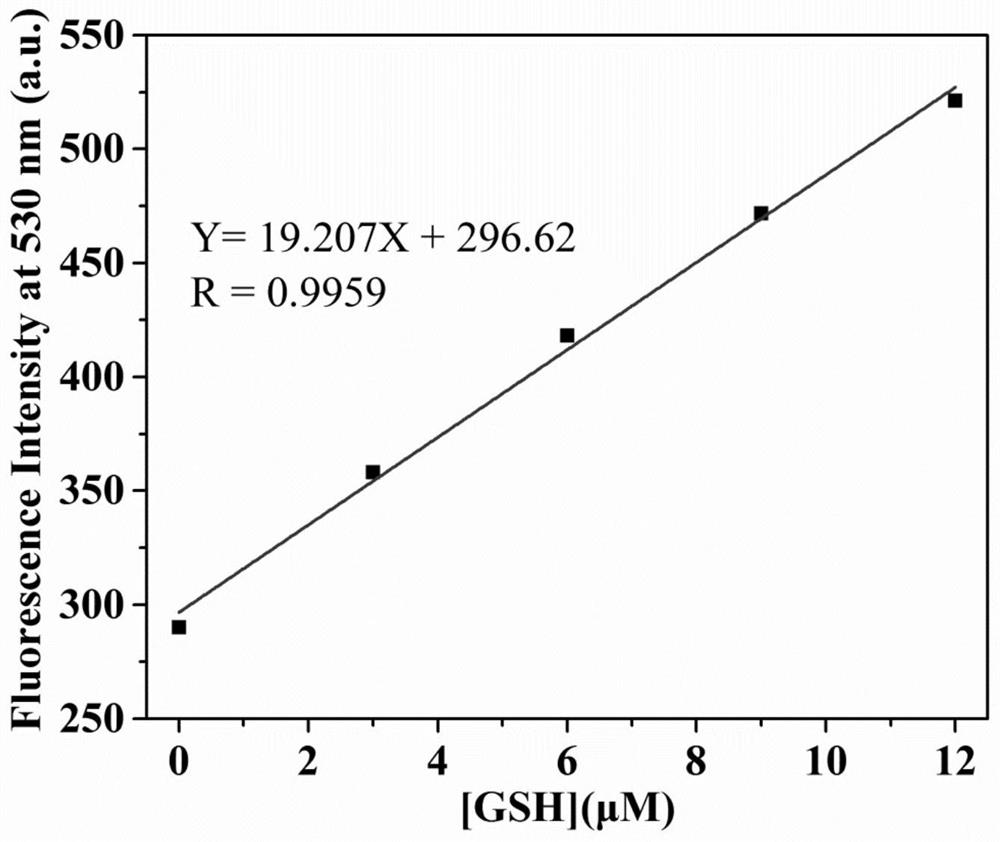
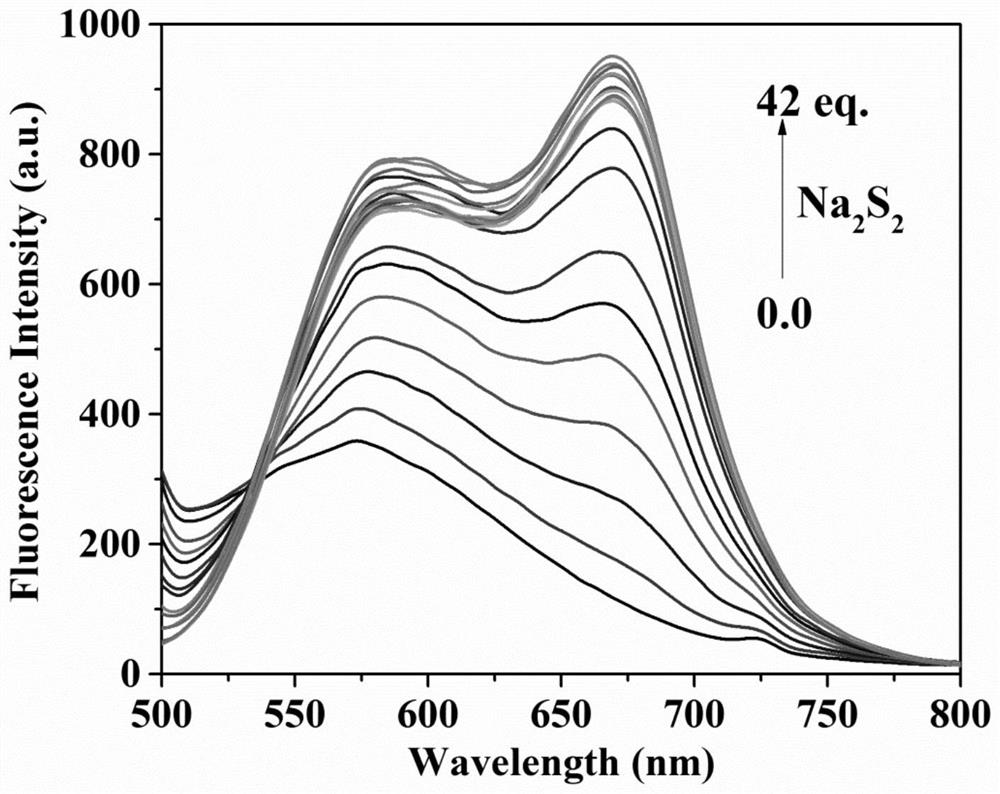
Fluorescent probe for differentially detecting GSH and H2Sn (n is more than) through single-wavelength excitation
A fluorescent probe, single-wavelength technology, applied in the field of chemical analysis and detection, can solve the problem of short emission wavelength of fluorescent probes, and achieve the effect of wide application range and good cell membrane penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of compound 2
[0040] Resorcinol (7.707 g, 70.0 mmol) and compound 1 (0.979 g, 3.5 mmol) were dissolved in 10.0 mL of acetonitrile. The mixture was stirred at 0°C for 5 minutes. Sodium chlorite (1.112 g, 12.3 mmol) and sodium dihydrogen phosphate (1.896 g, 15.8 mmol; dissolved in 1.5 mL of water) were added to the above mixture. After stirring and reacting at 0°C for 30 minutes, it was poured into 50 mL of ice water. The mixture was acidified with 1mol / L hydrochloric acid solution to form a large amount of yellow precipitate. After suction filtration and washing, 0.751 g of a yellow-brown solid was compound 2, and the yield was 76.2%.
Embodiment 2
[0041] Embodiment 2: the synthesis of compound 3
[0042] Diphenyldiselenide (0.218 g, 0.7 mmol) and sodium borohydride (0.034 g, 0.9 mmol) were dissolved in 2.5 mL of absolute ethanol. Under the protection of nitrogen, the mixture was stirred and reacted at room temperature for 10 minutes to obtain compound 3 as a colorless transparent solution. The product of this step can be directly used in the next step of synthesis without further purification.
Embodiment 3
[0043] Embodiment 3: the synthesis of compound 4
[0044] Compound 2 (0.180 g, 0.6 mmol) and compound 3 (0.110 g, 0.7 mmol) were dissolved in 5.0 mL of anhydrous N,N-dimethylformamide. And triethylamine (240.0 μL, 1.8 mmol) was added as an acid-binding agent. The reaction was stirred at room temperature for 20 minutes, then the mixture was poured into 50 mL of water. and extracted with dichloromethane, and the organic layers were combined. The organic solvent was removed under reduced pressure to obtain 0.243 g of orange solid as compound 4 with a yield of 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com