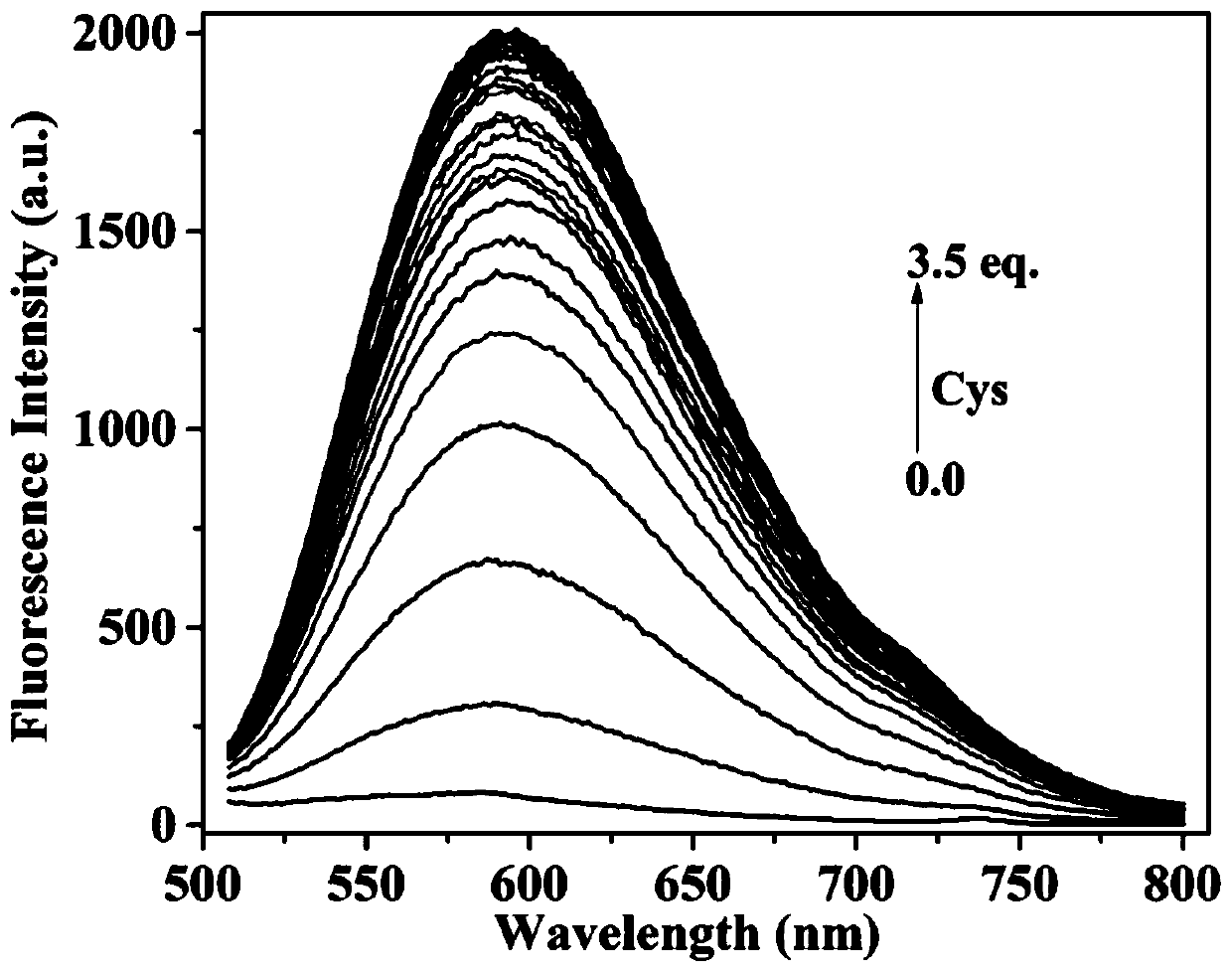
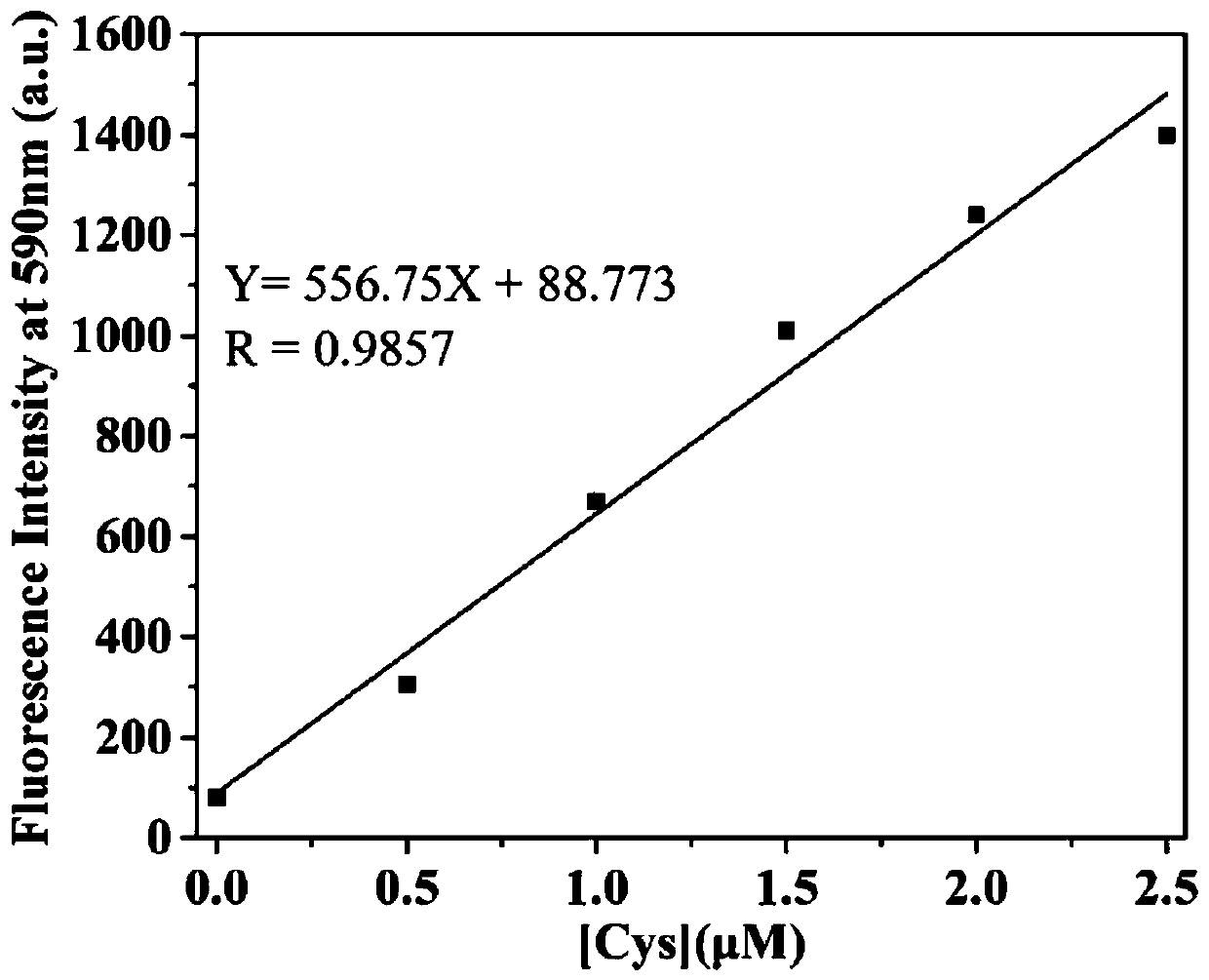
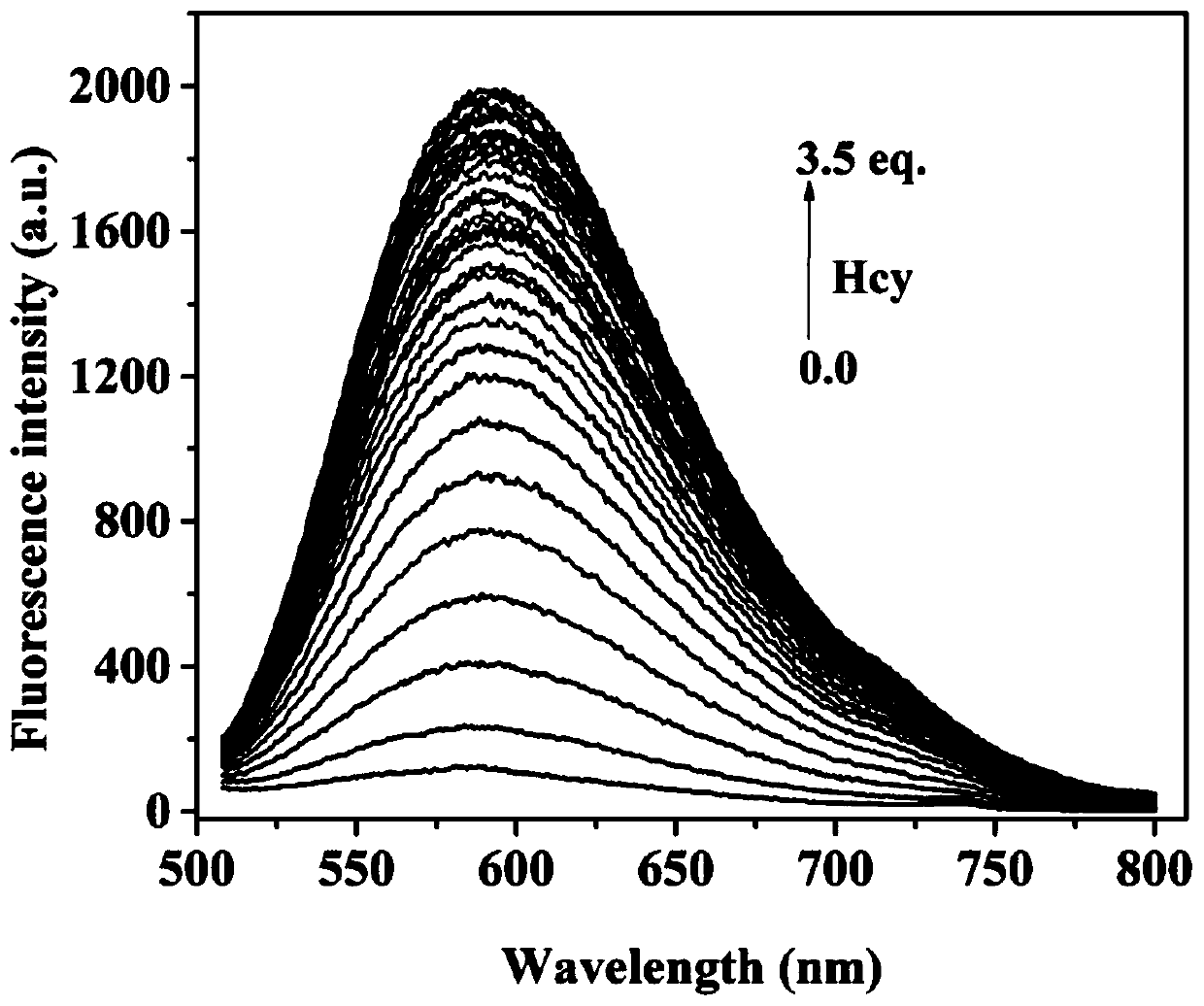
Fluorescent probe for identifying mercaptoamino-acid and preparation method of fluorescent probe
A fluorescent probe and identification technology, applied in the field of chemical analysis and detection, to achieve the effects of good selectivity, good cell membrane penetration, and strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of compound 2
[0040] Compound 1 (3.32g, 10.0mmol) was dissolved in 50mL of ethylene glycol monomethyl ether, heated to 115°C until the reaction solution became clear, and then 3.0mL of hydrazine hydrate (80%) was added dropwise to the above mixed solution. After the addition was complete, the mixture was heated to reflux for 3 hours. After the reaction was completed, it was cooled to room temperature, and a large amount of orange precipitate was precipitated, which was filtered and washed to obtain compound 2. Yield: 2.32 g; Yield: 81.9%.
Embodiment 2
[0041] Embodiment 2: the synthesis of compound 3
[0042] Intermediate 2 (1.98g, 7mmol) was dissolved in 3-methyl-2-butanone (100mL), and then 3mL of concentrated sulfuric acid was slowly added dropwise, and the reaction mixture was refluxed and stirred for 4 hours under nitrogen protection. After the reaction was completed, , 3-methyl-2-butanone was removed by rotary evaporation under reduced pressure to obtain a crude product, which was separated and purified by silica gel column chromatography (petroleum ether / dichloromethane=1 / 10, v / v) to obtain product 3 as a pale yellow solid. Yield: 1.13 g; Yield: 48.5%.
Embodiment 3
[0043] Embodiment 3: the synthesis of compound 4
[0044] Compound 3 (1.0g, 3mmol) and p-Hydroxybenzaldehyde (0.37g, 3mmol) were dissolved in 20mL of dry toluene, stirred and mixed, and then glacial acetic acid (0.02mL) and piperidine (0.05mL) were added dropwise to the above mixture solution, after the dropwise addition, the mixed solution was refluxed and stirred for 6 hours under the protection of nitrogen, and the toluene was evaporated to dryness under reduced pressure to obtain a crude product, which was separated and purified by column chromatography (ethanol / dichloromethane=1 / 50, v / v ) to obtain product 4 as a red solid. Yield: 0.68 g; Yield: 51.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com