Cavity transfer material with triphenylamine-bipyridyl structure and preparation method thereof
A hole transport material, triphenylamine technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve problems such as unfavorable commercial applications, poor film-forming properties of small molecular compounds, etc., to expand design ideas, The effect of good fluorescence lifetime and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
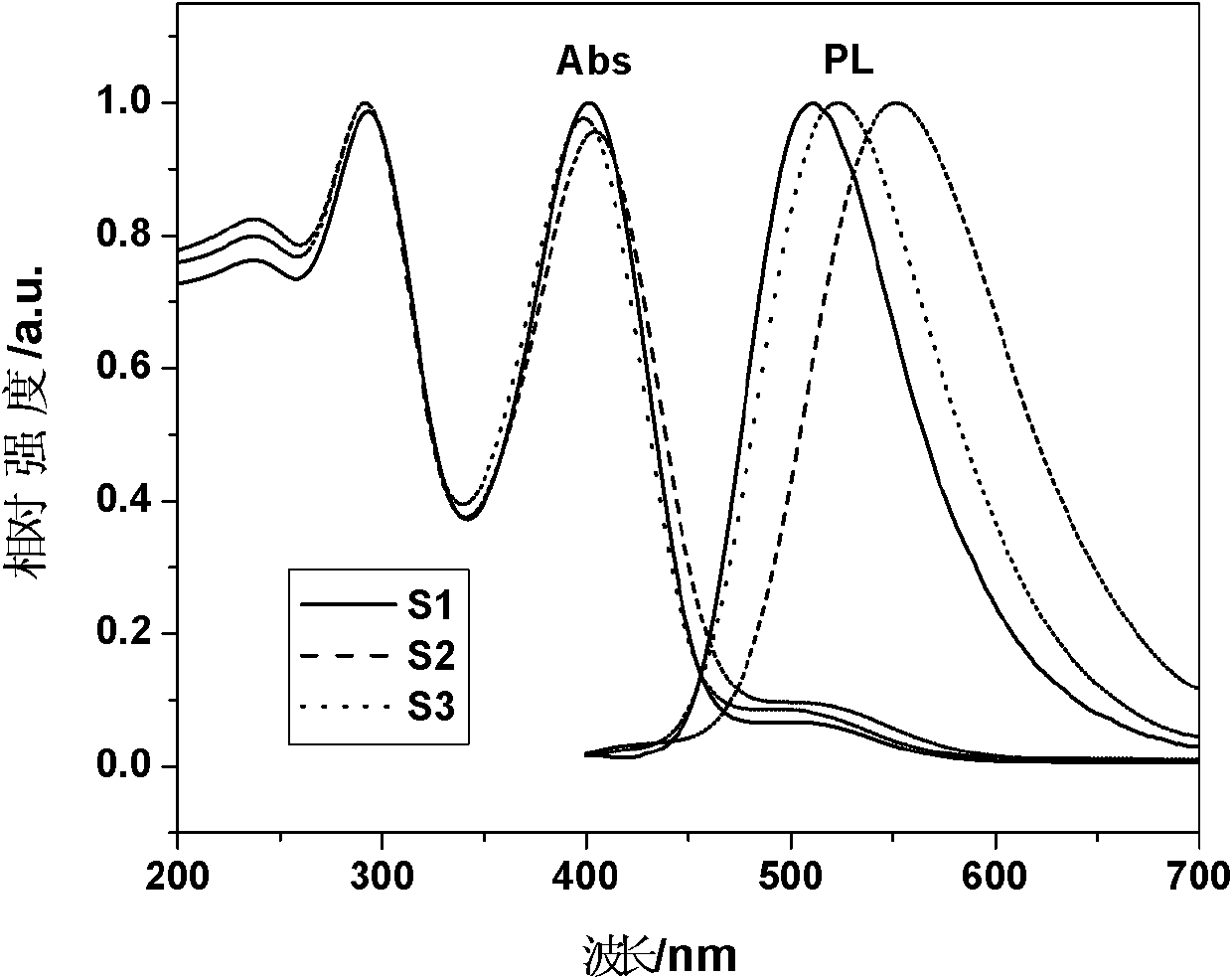
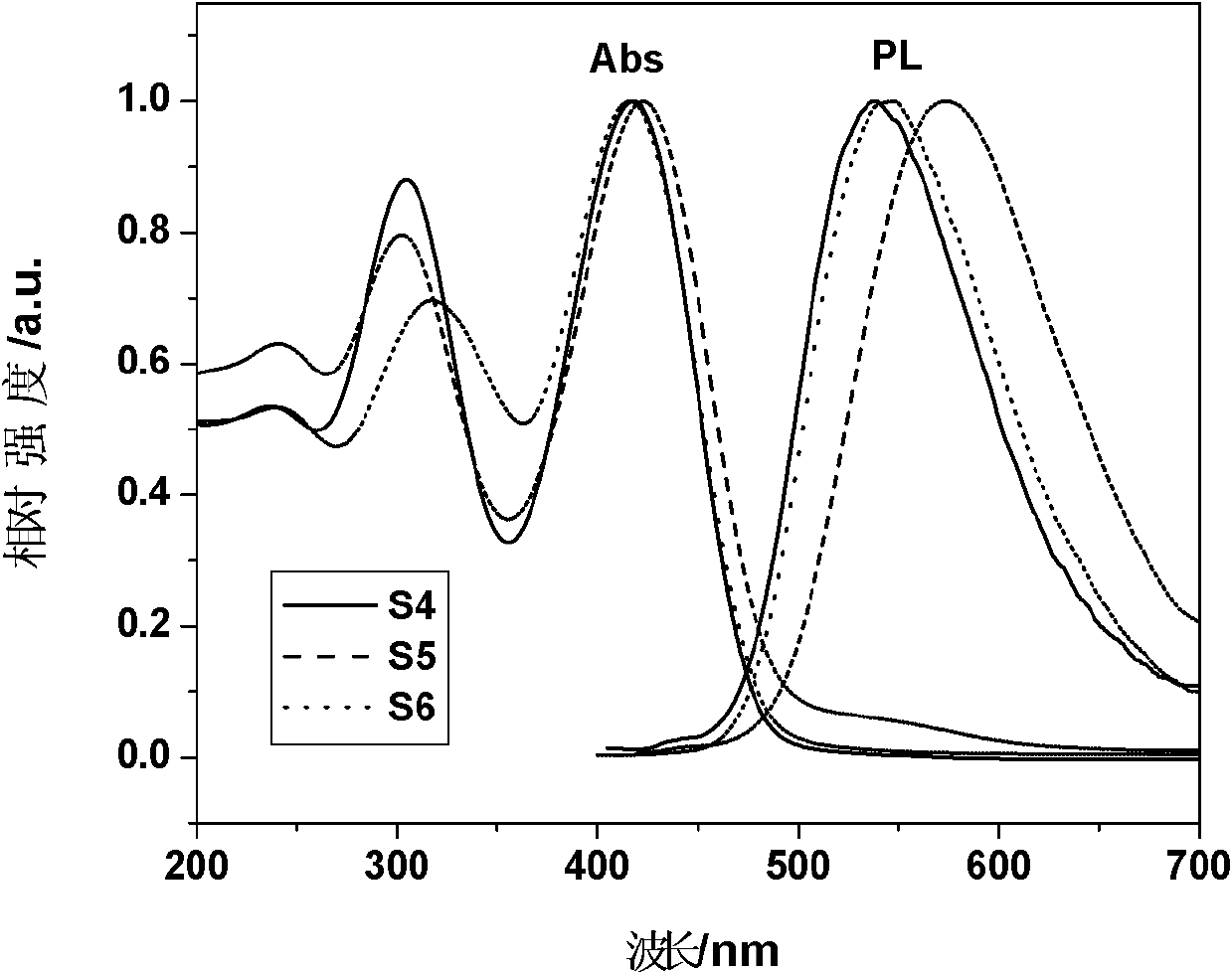
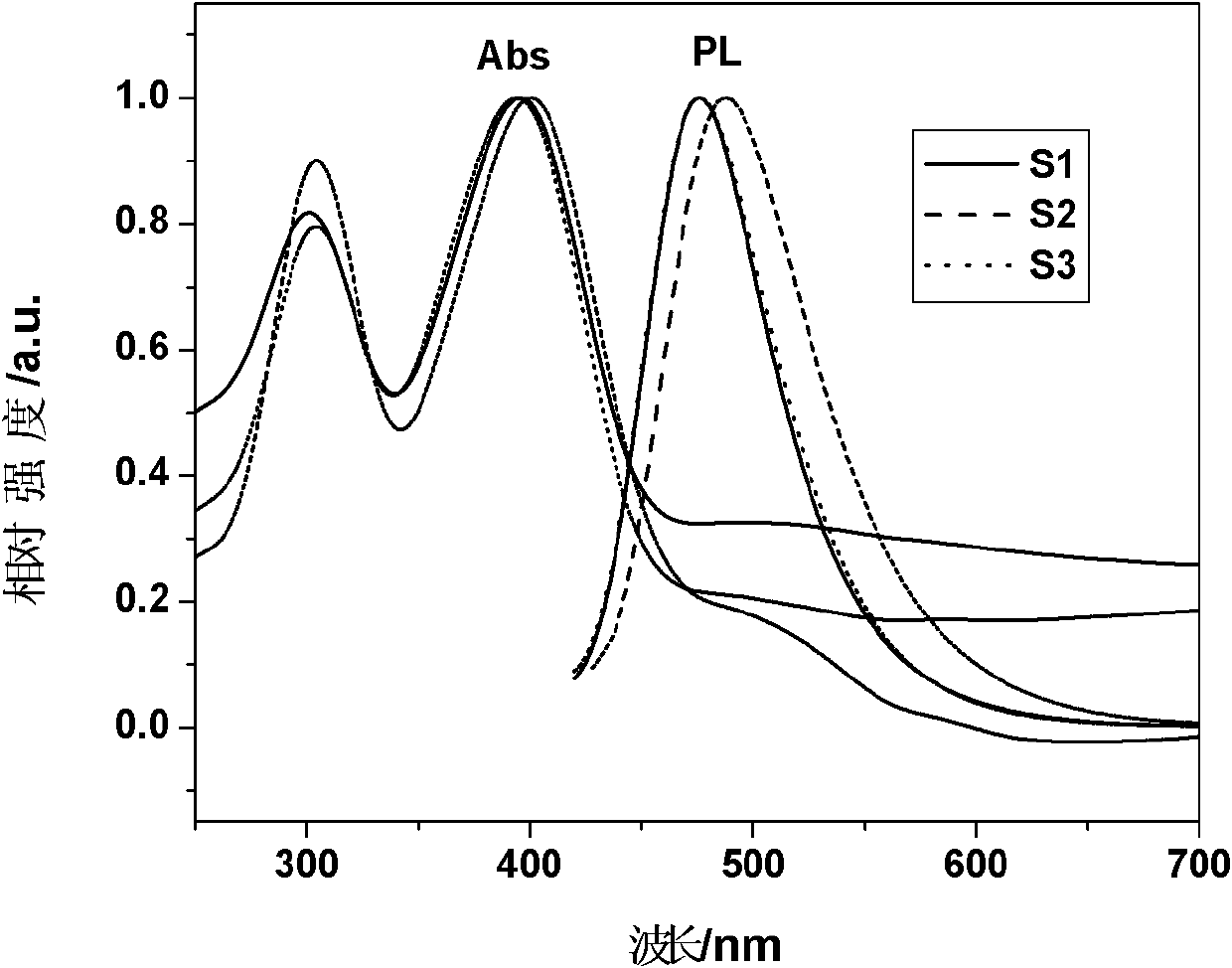
Image
Examples
Embodiment 1~3
[0047] Examples 1-3 are 4-methyl-4'-(2-(4-(N,N-dimethylphenyl)amino)phenyl)vinyl-2,2'-bipyridine (S1)
[0048] The synthetic route is as follows:
[0049]
Embodiment 1
[0050] Example 1: Synthesis of 4-methyl-4'-(2-(4-(N,N-di-p-methylphenyl)amino)phenyl)vinyl-2,2'-bipyridine (S1)
[0051] N 2 Under protection, add 0.42g (1.35mmol) triphenylamine aldehyde compound 1, 0.48g (1.5mmol) Horner reagent (compound 7) and 60mL dry tetrahydrofuran to a 250mL four-neck flask, stir until completely dissolved, then cool down in an ice bath to At 0°C, 0.33 g (3 mmol) of potassium tert-butoxide in tetrahydrofuran was slowly added dropwise, and the molar ratio of compound 1, Horner's reagent and potassium tert-butoxide was 1:1.1:2.2. The reaction solution was stirred for 6 hours in an ice bath, then the solvent was removed by rotary evaporation, dissolved in dichloromethane, washed twice with water, and washed with anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation, and the crude product was separated by column chromatography (cyclohexane / ethyl acetate=5:1) to obtain 0.44 g of the target product S1, with a yield of 70%. 1 H NMR (C...
Embodiment 2
[0052] Example 2: Synthesis of 4-methyl-4'-(2-(4-(N,N-di-p-methylphenyl)amino)phenyl)vinyl-2,2'-bipyridine (S1)
[0053] N 2 Under protection, add 0.42g (1.35mmol) triphenylamine aldehyde compound 1, 0.48g (1.5mmol) Horner reagent (compound 7) and 60mL dry tetrahydrofuran to a 250mL four-neck flask, stir until completely dissolved, then cool down in an ice bath to At 0°C, 0.25 g (2.25 mmol) of potassium tert-butoxide in tetrahydrofuran was slowly added dropwise. The molar ratio of compound 1, Horner's reagent and potassium tert-butoxide was 1:1.1:1.65. The reaction solution was stirred for 8 hours in an ice bath, then the solvent was removed by rotary evaporation, dissolved in dichloromethane, washed twice with water, and then washed with anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation, and the crude product was separated by column chromatography (cyclohexane / ethyl acetate=5:1) to obtain 0.38 g of the target product S1 with a yield of 61%. 1 H NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com