Method of synthesizing imine and amine compounds by means of borrowing-hydrogen reduction coupling
A technology of amine compounds and compounds, which is applied in the preparation of amino compounds, organic compounds, and imino compounds through condensation/addition reactions. It can solve the problems of expensive precious metals, limited earth reserves, and limited applications. Inexpensive, easy to operate, mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of nitrogen-doped porous carbon material-supported Co catalyst
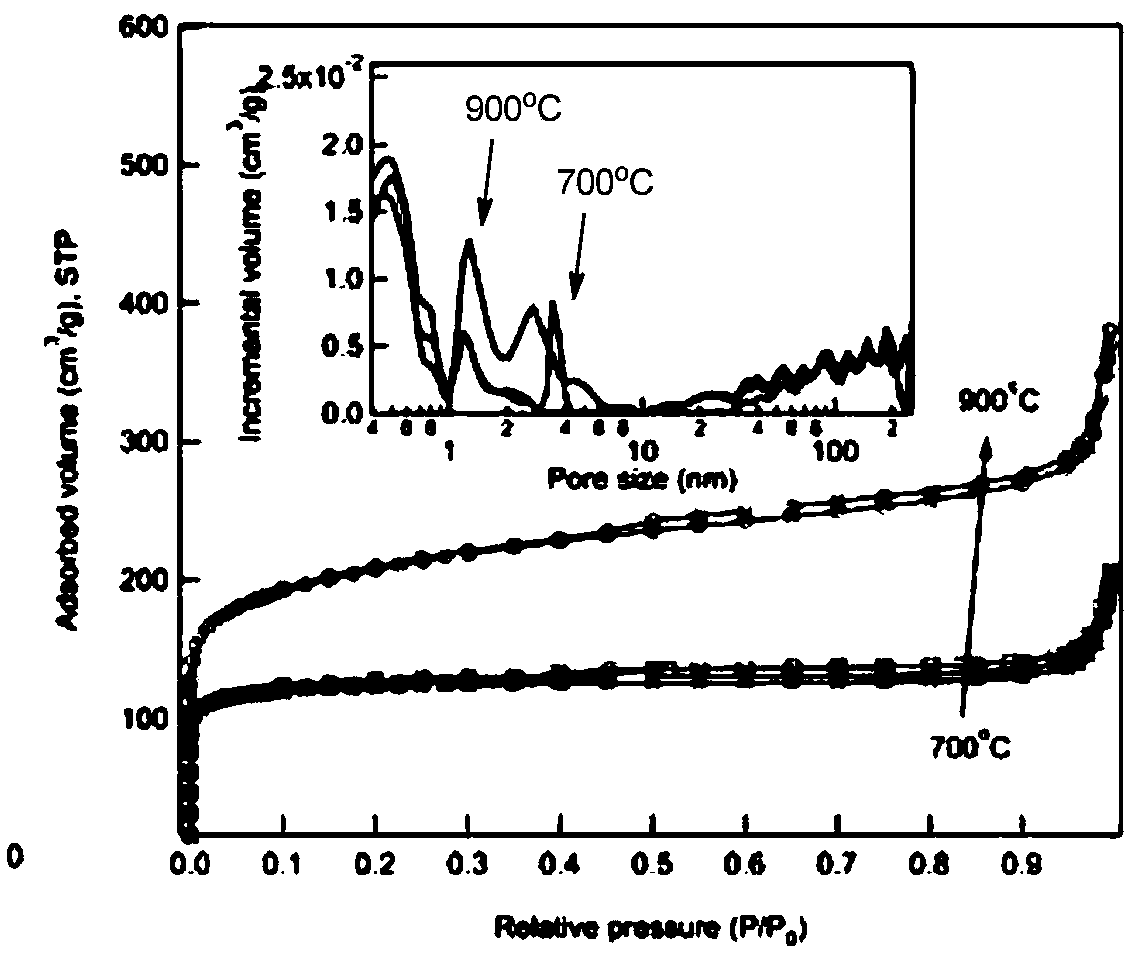
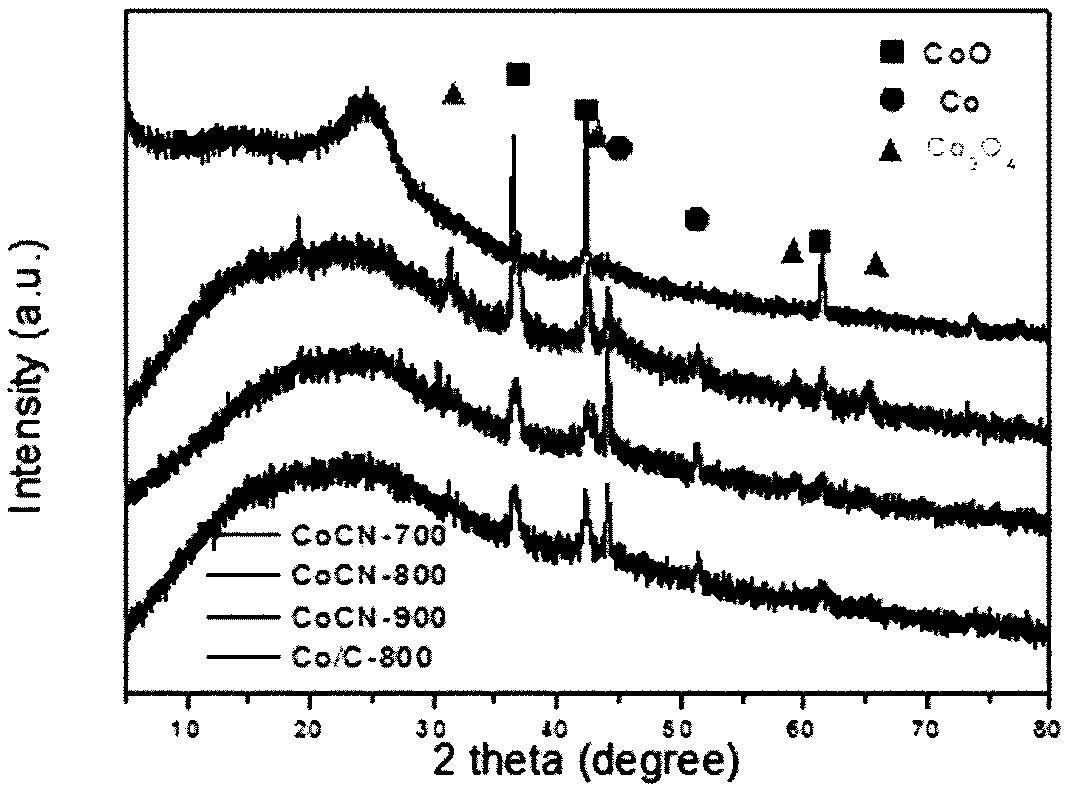
[0041] Cut 1kg of cleaned bamboo shoots into pieces, heat them in an oven at 70°C until dry, grind the dried solid into powder, and put it into powder for later use; add 2g of the powder into 15mL of water, stir and mix evenly, and then transfer it to a hydrothermal reaction kettle. React at 180°C for 8 hours, filter after the reaction, wash the filtered product with water, and dry to obtain a brown solid, vacuum-dry the obtained solid for 24 hours, and grind until the particles are uniform to obtain hydrothermal carbon. After that, 0.5 g of brown solid hydrothermal carbon obtained above was dispersed in 0.0495 g of CoCl 2 ·6H 2 O in 15mL of water, stirred at 60°C for 2h, dried the reaction solution at 100°C for 12h, then put the obtained dry solid in a tube furnace for calcination in a nitrogen gas atmosphere, and kept it at 800°C for 2 hours , after the tube furnace dropped to ro...
Embodiment 2
[0044] The invention discloses a method for preparing imine compounds by hydrogen-reductive coupling of nitroaromatic compounds and differently substituted benzyl alcohol compounds using nitrogen-doped carbon material supported cobalt catalysts. The steps are:
[0045] Add 0.2mmol of nitroaromatic compounds, 0.6mmol of different substituted benzyl alcohol compounds, 20mg of the supported catalyst, 5mL of toluene, 67.5mg (0.6mmol) of potassium tert-butoxide into the reaction tube. Carry out the reaction, after reacting for 15 hours, cool to room temperature, filter the reaction solution, and perform silica gel column chromatography to obtain imine compounds;
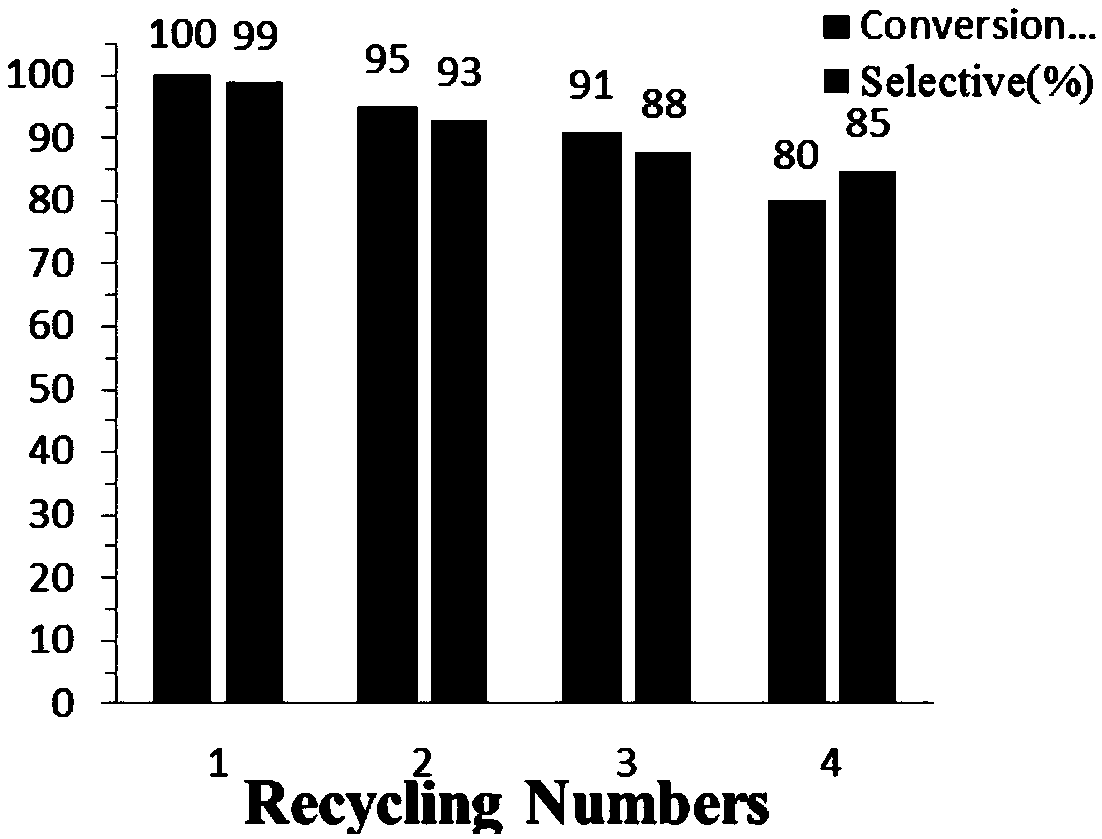
[0046] Same operation and steps as in Example 2, changing the types of nitro compounds and differently substituted benzyl alcohol compounds (i.e. substrates), the obtained imine compounds (products), conversions > 99%, and yields of 60-90% The % varies, as shown in Table 1:
[0047] Table 1
[0048]
[0049]
Embodiment 22
[0051] The invention discloses a method for preparing amine compounds through hydrogen reductive coupling of nitroaromatic compounds and differently substituted benzyl alcohol compounds by using nitrogen-doped carbon material supported cobalt catalyst to catalyze. The steps are:
[0052] Add 0.2mmol of nitroaromatic compounds, 0.8mmol of differently substituted benzyl alcohol compounds, 20mg of the supported catalyst, 5mL of toluene, 89.6mg (0.8mmol) of potassium tert-butoxide into the reaction tube, and seal it at 120°C Carry out the reaction, after reacting for 15 hours, cool to room temperature, filter the reaction solution, and obtain the imine compound by silica gel column chromatography; the same operation and steps as in Example 23, change the nitro compound and differently substituted benzyl alcohol compounds (i.e. The kind of substrate), the amine compound (product) that obtains, transformation rate all>99%, productive rate 60~90% differ, specifically as shown in tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com