Method for synthesizing hypericin
A technology of hypericin and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of quinones, etc., can solve the problems of long reaction time, large environmental pollution, difficult purification of products, etc., and achieves relative yield and The effect of high purity, less environmental pollution and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The following are specific examples of the present invention, and further describe the technical solution of the present invention, but the protection scope of the present invention is not limited to these examples. All changes or equivalent substitutions that do not depart from the concept of the present invention are included in the protection scope of the present invention.
[0020] The methods used in the following examples are conventional methods unless otherwise specified.
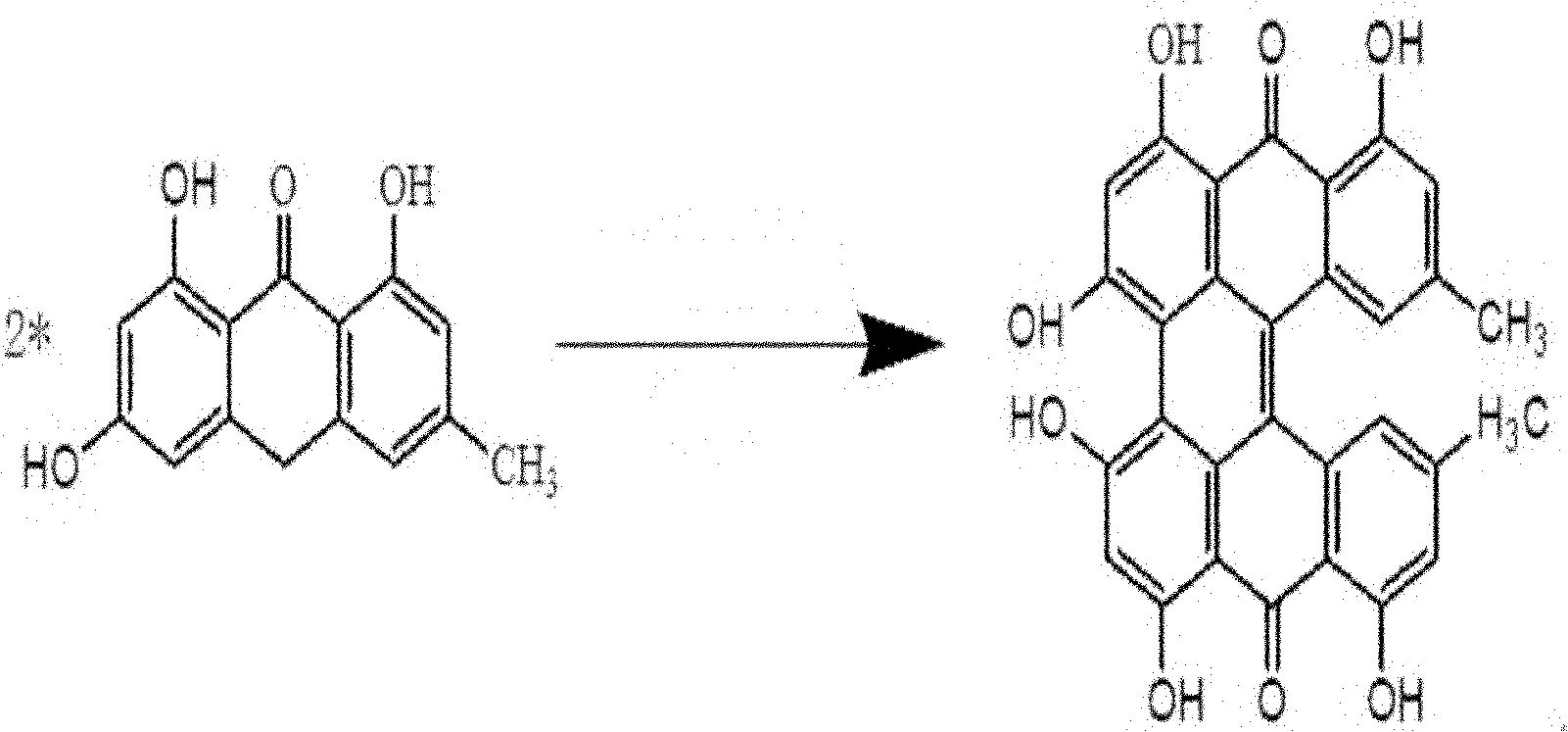
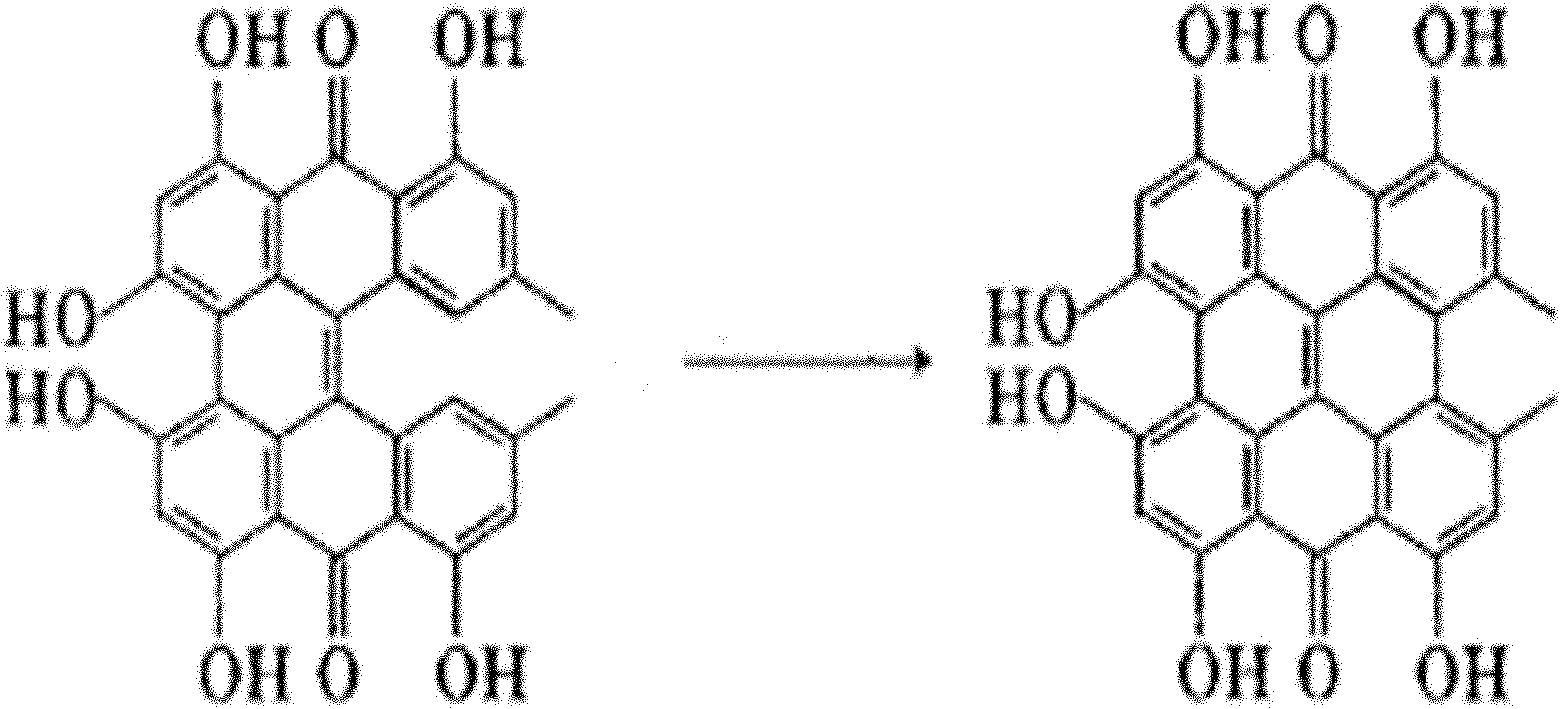
[0021] Now use the method artificial synthesis hypericin of the present invention, concrete example comprises the following steps:
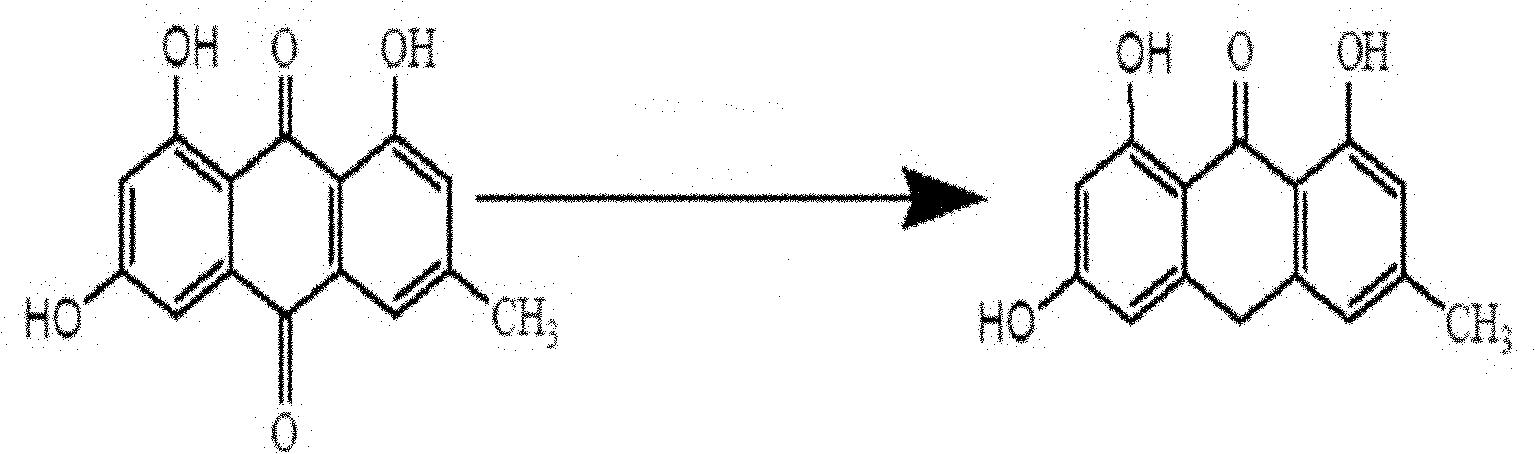
[0022] (1) Convert emodin into emodin anthrone
[0023] Get emodin 2g (7.4mmol), add in glacial acetic acid 120mL, Ar 2 protection, add SnCl under stirring 2 2H 2 O2.8g (12.4mmol), the mixture began to be heated, the reaction temperature reached 115°C, and reflux began to occur. At about 110°C, 48 mL of concentrated hydrochloric acid was added dropwise, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com