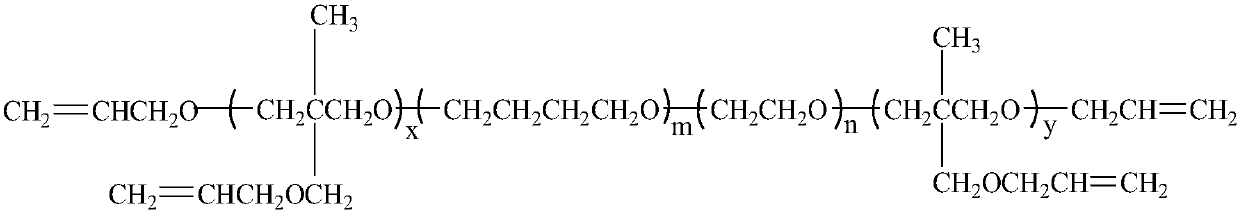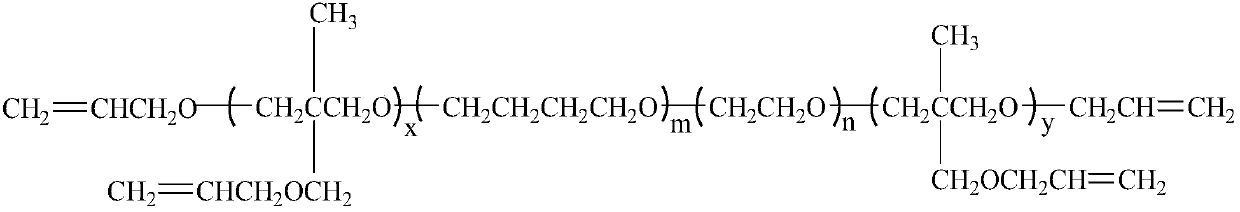Polyalkenyl-terminated copolyether bonding agent and synthesis method thereof
A technology of alkenyl copolyether and synthesis method, which is applied in the direction of polyether adhesives, adhesive types, adhesives, etc., can solve the problems of harsh curing conditions, high curing temperature, and water sensitivity, so as to improve regularity, The effect of high mechanical properties and high flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
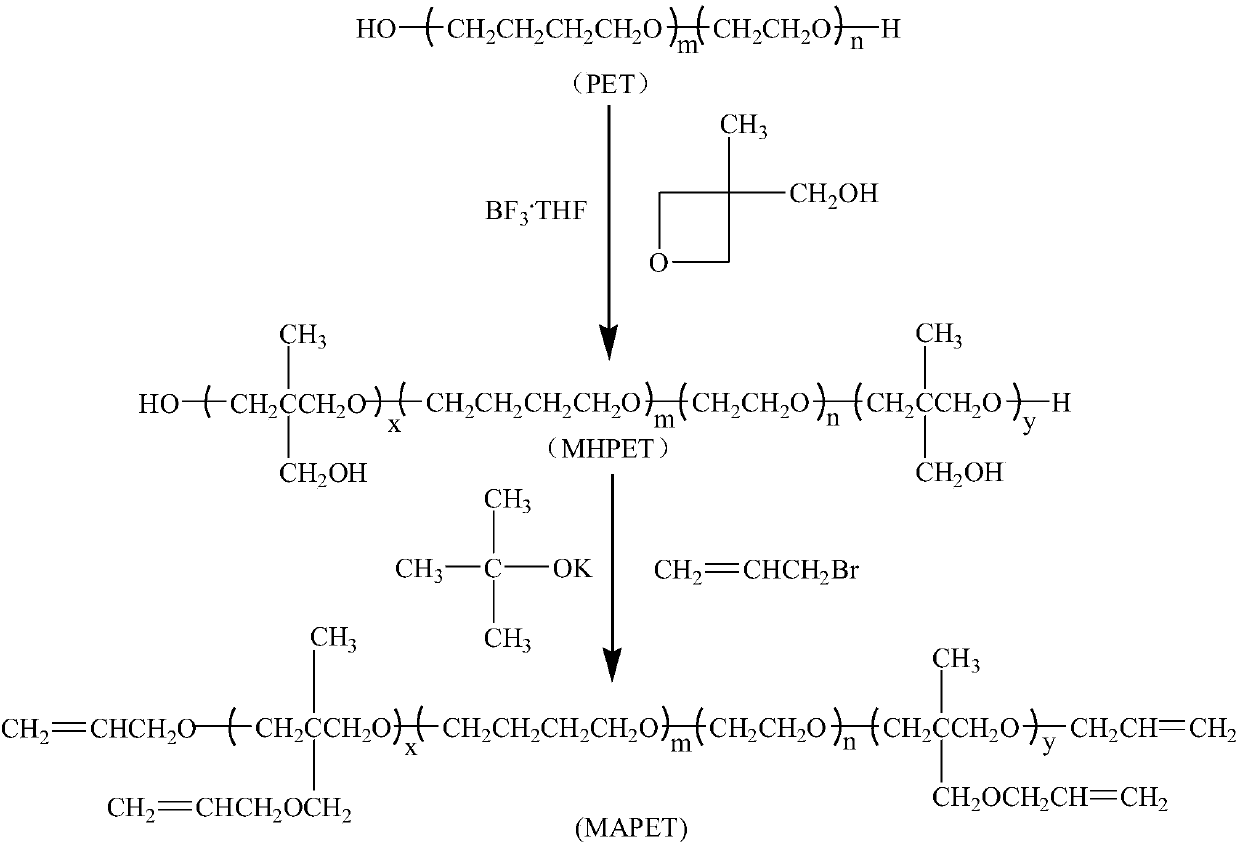
[0028] (1) Synthesis of MHPET
[0029] In a 250ml four-necked flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, 50g (0.02mol) of hydroxyl-terminated oxirane-tetrahydrofuran copolyether (PET), 100ml of dichloromethane and 1.68g Boron trifluoride·tetrahydrofuran complex, stirred for 0.5h, cooled to 2°C, began to slowly add 4.08g of 3-hydroxymethyl-3-methyloxetane dropwise, the dropping time was 4h, and the dropwise addition was completed After that, continue the insulation reaction for 24h, and use Na 2 CO 3 The aqueous solution was neutralized, then washed 3 times with distilled water, poured into a separatory funnel, the organic phase was separated, and the solvent was evaporated under reduced pressure to obtain 52.7 g of light yellow viscous transparent liquid with a yield of 97.4%.
[0030] Structural identification: IR, ν max (cm -1 ): 3440(-OH), 2846, 2965(-CH 3 、-CH 2 ), 1101 (C-O-C).
[0031] 1 H NMR (CDCl 3 , 500MHz): 1...
Embodiment 2
[0040] (1) Synthesis of MHPET
[0041] In a 250ml four-neck flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, 50g (0.02mol) of hydroxyl-terminated oxirane-tetrahydrofuran copolyether (PET), 100ml of dichloromethane and 2.24g Boron trifluoride·tetrahydrofuran complex, stirred for 0.5h, cooled to 3°C, began to slowly add 6.12g of 3-hydroxymethyl-3-methyloxetane dropwise, the dropping time was 5h, and the dropwise addition was completed After that, continue the insulation reaction for 28h, and use Na 2 CO 3 The aqueous solution was neutralized, then washed 3 times with distilled water, poured into a separatory funnel, the organic phase was separated, and the solvent was evaporated under reduced pressure to obtain 54.5 g of light yellow viscous transparent liquid with a yield of 97.1%.
[0042] The number average molecular weight is 2810, and the hydroxyl value is 96.8 mgKOH / g.
[0043] (2) Synthesis of MAPET
[0044] In a 250ml four-n...
Embodiment 3
[0047] (1) Synthesis of MHPET
[0048] In a 250ml four-necked flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, 60g (0.02mol) of hydroxyl-terminated oxirane-tetrahydrofuran copolyether (PET), 100ml of dichloromethane and 2.24g Boron trifluoride tetrahydrofuran complex, stirred for 0.5h, cooled to 4°C, began to slowly add 6.12g of 3-hydroxymethyl-3-methyloxetane dropwise, the dropping time was 5h, and the dropwise addition was completed After that, continue the insulation reaction for 28h, and use Na 2 CO 3 The aqueous solution was neutralized, then washed three times with distilled water, poured into a separatory funnel, the organic phase was separated, and the solvent was evaporated under reduced pressure to obtain 64.5 g of light yellow viscous transparent liquid with a yield of 97.5%.
[0049] The number average molecular weight was 3320, and the hydroxyl value was 83.1 mgKOH / g.
[0050] (2) Synthesis of MAPET
[0051] In a 250m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com