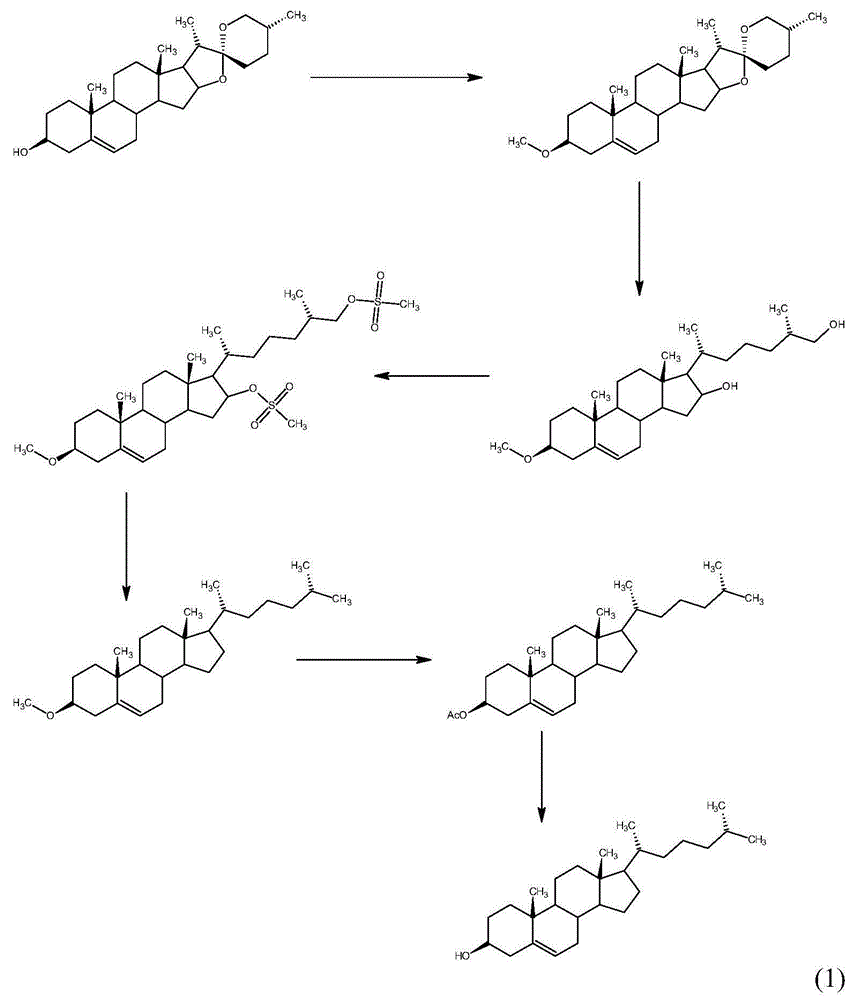
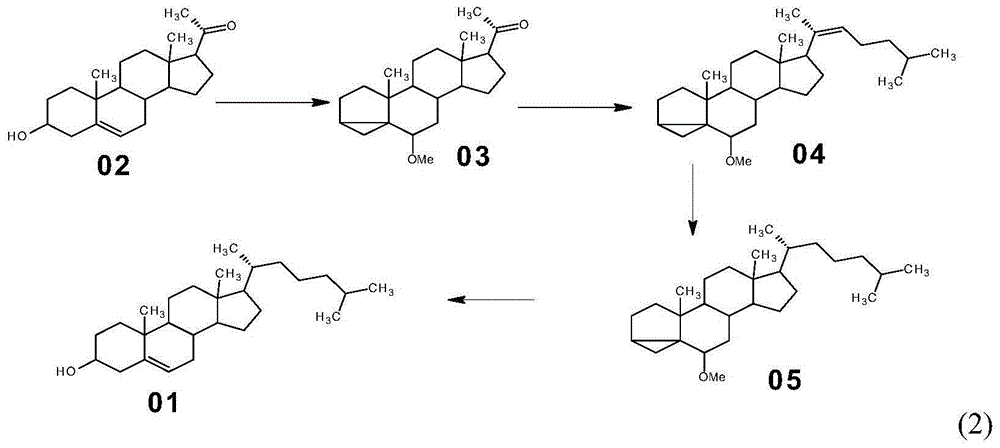
Method for synthesizing cholesterol by using pregnenolone as raw material
A technology of pregnenolone and cholesterol, applied in the direction of steroids, organic chemistry, etc., can solve the problems of large consumption of raw and auxiliary materials, uneconomical, heavy pollution, etc., and achieve the effect of low production cost, less raw and auxiliary materials, and environmentally friendly processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound (03)
[0035] Add pyridine 500mL, pregnenolone (02) 100g, benzenesulfonyl chloride 61g to a 1000mL reaction flask, stir and react at room temperature for 24 hours, then pour the reaction solution into a large amount of ice water, a large amount of solid is precipitated, filter, and filter the cake with clean water Wash and dry to obtain the intermediate sulfonate wet product. Dissolve the wet product of the sulfonate in 1000 mL of methanol, add 93 g of potassium acetate, heat the reaction mixture to reflux, react under reflux for 2 hours, stop heating and reflux; concentrate under reduced pressure to about 200 mL of methanol, freeze and crystallize to obtain the compound ( 03) 96.00g, molar yield 91.93%;
Embodiment 2
[0036] The synthesis of embodiment 2 compound (03)
[0037] The synthetic method is the same as in Example 1, except that in this example, the consumption of benzenesulfonyl chloride and potassium acetate in Example 1 is adjusted to 67g and 124g respectively.
[0038] 96.36 g of compound (03) was obtained, and the molar yield was 92.27%.
Embodiment 3
[0039] The synthesis of embodiment 3 compound (03)
[0040] The synthetic method is the same as in Example 1, except that in this example, the consumption of benzenesulfonyl chloride and potassium acetate in Example 1 is adjusted to 73g and 155g respectively.
[0041] 95.72 g of compound (03) was obtained, and the molar yield was 91.66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com