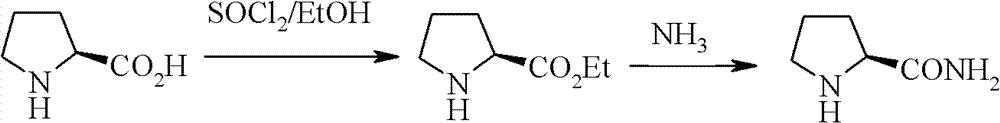
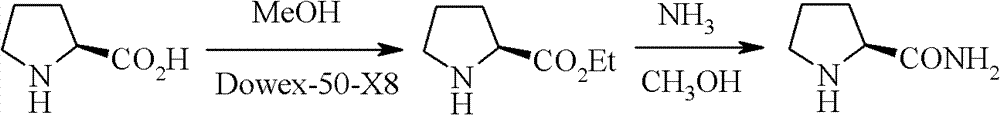
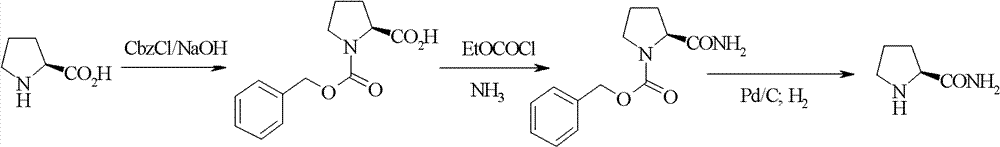
New synthetic method of high-optical activity prolinamide
A technology of prolinamide and optical activity, which is applied in the synthesis of organic compounds and the synthesis of high optical chiral prolinamide, which can solve the problems of less side reactions, high yield, racemization, etc., and achieve low cost and easy industrialization Operation, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of N-Benzyloxycarbonyl-D-Proline Methyl Ester
[0025] Put 330 g of D-proline methyl ester into 2.4 L of water, cool in an ice-water bath, and simultaneously add 500 g of benzyl chloroformate and 10% aqueous sodium hydroxide solution dropwise, and control the temperature in the reaction system at 5 to 15 degrees. The pH value is 8±0.5. Continue to react for 3 hours after dropping. until the reaction is complete. The organic phase was extracted twice with 500 mL of dichloromethane. Combine the organic phases. Dry over anhydrous sodium sulfate. The solvent was removed to obtain a light yellow oily liquid.
[0026] Synthesis of N-Benzyloxycarbonyl-D-prolineamide
[0027] Dissolve the N-benzyloxycarbonyl-D-proline methyl ester obtained in the previous step in 2000 mL of methanol, and cool to 0°C with an ice-water bath. Ammonia gas is introduced and a pressure of 1.5-2 kg is maintained. React for 5 hours, heat up to 50 degrees, and react for 2 hours. After...
Embodiment 2
[0031] Synthesis of N-Benzyloxycarbonyl-D-Proline Methyl Ester
[0032] Put 330 g of D-proline methyl ester into 2.4 L of water, cool in an ice-water bath, and simultaneously add 654 g of benzyl chloroformate and 10% aqueous sodium hydroxide solution dropwise, and control the temperature in the reaction system at 30-45 degrees. The pH value is 9±0.5. Continue to react for 3 hours after dropping. until the reaction is complete. The organic phase was extracted twice with 500 mL of dichloromethane. Combine the organic phases. Dry over anhydrous sodium sulfate. The solvent was removed to obtain a yellow oily liquid.
[0033] Synthesis of N-Benzyloxycarbonyl-D-prolineamide
[0034] Dissolve the N-benzyloxycarbonyl-D-proline methyl ester obtained in the previous step in 2000 mL of ethanol, and cool to 0°C with an ice-water bath. Ammonia gas is introduced and a pressure of 3-4 kg is maintained. React for 5 hours, heat up to 65 degrees, and react for 2 hours. After the reacti...
Embodiment 3
[0038] Synthesis of N-Benzyloxycarbonyl-D-Proline Methyl Ester
[0039] Put 330 g of D-proline methyl ester into 2.4 L of water, and cool in an ice-water bath. At the same time, 741 g of benzyl chloroformate and 10% aqueous sodium hydroxide solution were added dropwise, and the temperature in the reaction system was controlled at -10-0 degrees. The pH value is 10±0.5. Continue to react for 3 hours after dropping. until the reaction is complete. The organic phase was extracted twice with 500 mL of dichloromethane. Combine the organic phases. Dry over anhydrous sodium sulfate. The solvent was removed to obtain a light yellow oily liquid.
[0040] Synthesis of N-Benzyloxycarbonyl-D-prolineamide
[0041] Dissolve the N-benzyloxycarbonyl-D-proline methyl ester obtained in the previous step in 2000 mL of isopropanol, and cool to 0°C with an ice-water bath. Introduce ammonia gas, and keep 4-5 kilograms of pressure. React for 2 hours, heat up to 80 degrees, and react for 2 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com