Lysine alpha-amino carbobenzoxy high-efficiency selective protection method and product thereof
A technology of benzyloxycarbonyl and lysine, applied in the field of efficient and selective protection of benzyloxycarbonyl, can solve the problems of multiple environmental pollutants, high treatment cost, harsh conditions, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
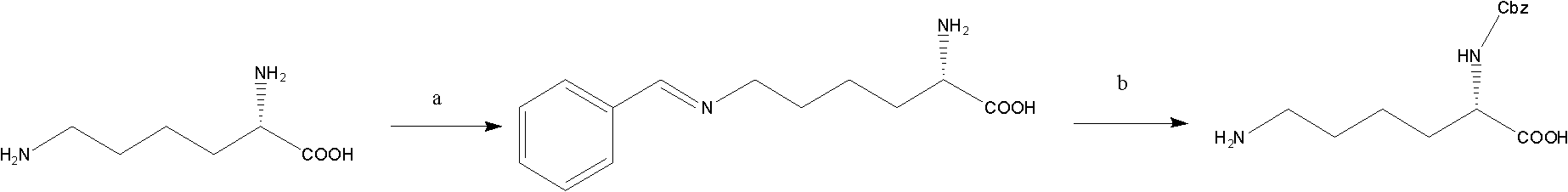
[0035] 11.35 g of β-cyclodextrin was dissolved in 113.5 g of purified water and adjusted to pH=8.5 with carbonate. 14.6 g of lysine were added to the above aqueous solution at 20°C. After the lysine is completely dissolved, add dropwise the benzyl chloroformate with a molar ratio of lysine:benzyl chloroformate=1:1.1 under the temperature control of the system at 19-21°C, and continue stirring for 10 minutes. The reaction solution was adjusted to pH=6.9 with hydrochloric acid, and extracted three times with 330 mL of ethyl acetate. The extracts were combined, and the solvent was distilled under the conditions of 30°C and 25mmHg pressure to obtain the lysine α-amino Cbz pre-protected product.
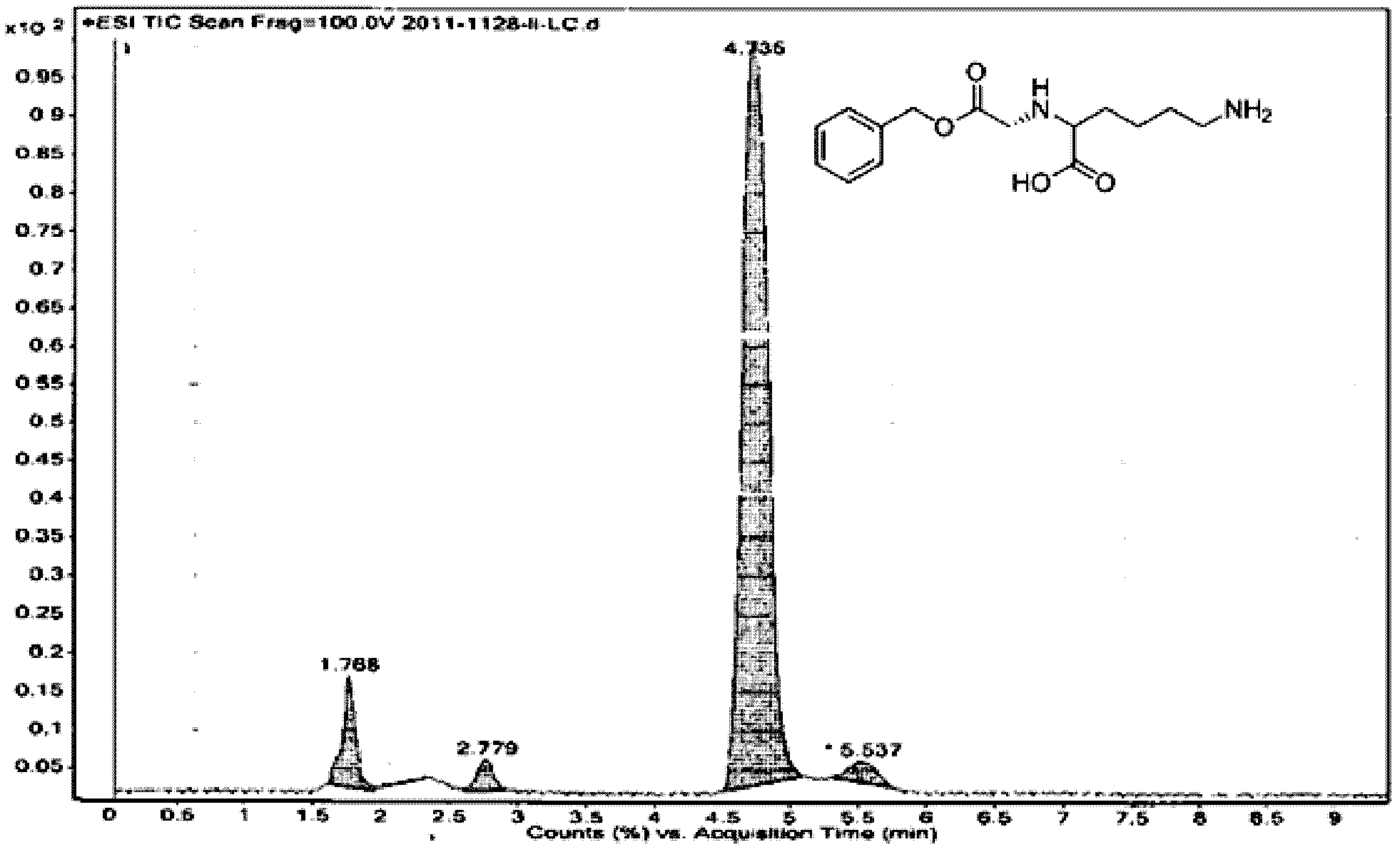
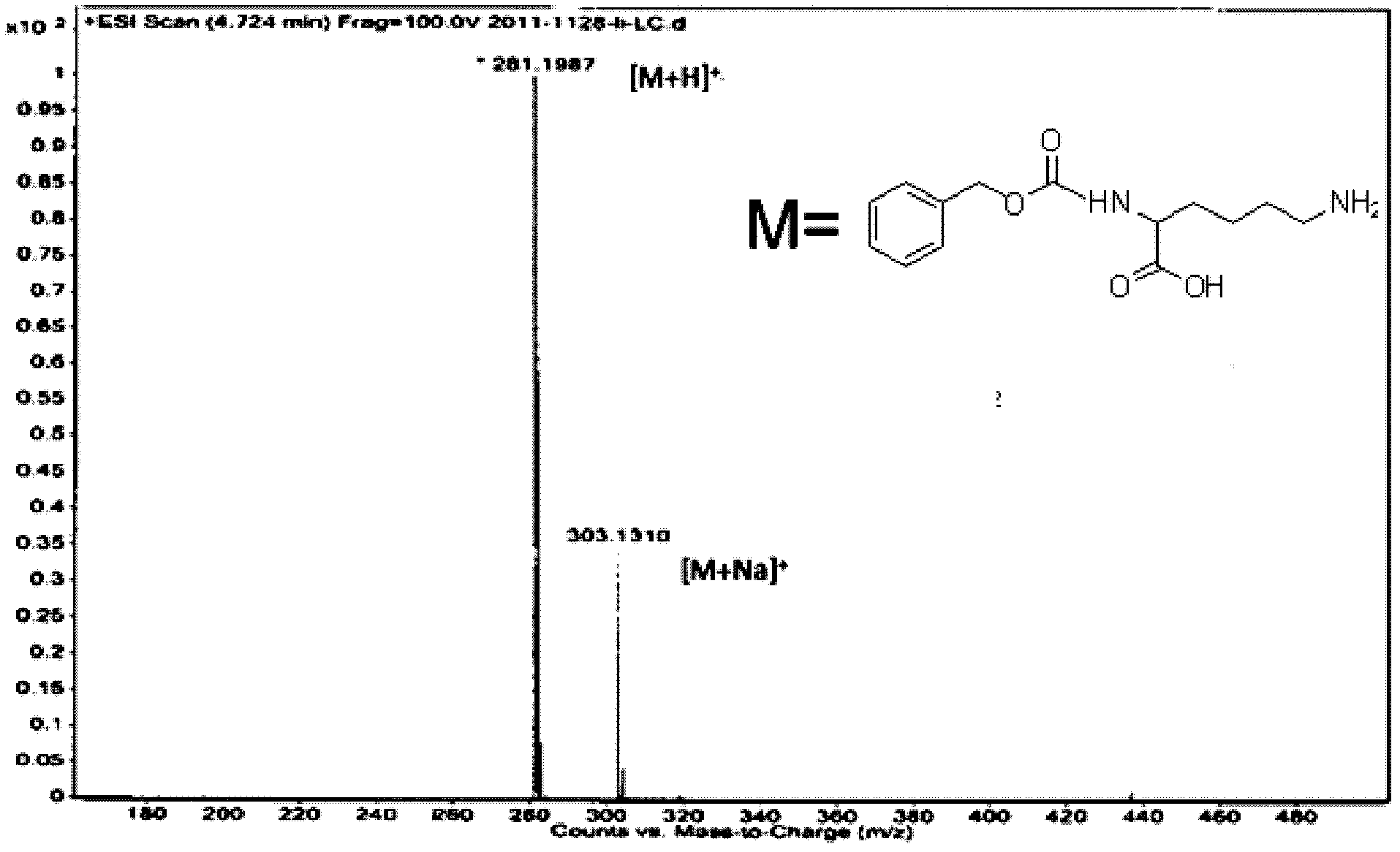
[0036] Physical and chemical data of the above product: melting point m.p.229℃~230℃ (literature value: m.p.227℃~231℃); mass spectrum (C 14 h 20 N 2 o 4 ): ESI-MS: 281.1 [M+H + ]; 303.1 [M+Na + ]; liquid chromatography-mass spectrometry data as shown in the figure (mobile phase: meth...
Embodiment 2
[0038] (1) In parts by weight (unit: kilogram), dissolve 30 parts of β-cyclodextrin in 100 parts of water, and adjust the pH to 8.5 with sodium hydroxide solution;
[0039] (2) Add lysine to the above solution at a molar ratio of β-cyclodextrin: lysine = 1:10 at 25°C, and stir until the lysine is completely dissolved;
[0040] (3) Add benzyl chloroformate dropwise to the solution of step (2) in a molar ratio of lysine: benzyl chloroformate=1:1.5, while controlling the reaction temperature at 20 ± 1°C, and continue stirring 20 minutes;
[0041] (4) the reaction solution is adjusted to neutrality with hydrochloric acid;
[0042] (5) step (4) solution is extracted 5 times with ethyl acetate;
[0043] (6) Combine the extracts, distill under reduced pressure at a temperature of 30° C. and a pressure of 25 mmHg to obtain the benzyloxycarbonyl pre-protected product of the α-amino group of lysine.
Embodiment 3
[0045] (1) In parts by weight (unit: kilogram), 40 parts of β-cyclodextrin was dissolved in 100 parts of water, and adjusted to pH=8.0 with sodium hydroxide solution;
[0046] (2) Add lysine into the above solution at a molar ratio of β-cyclodextrin: lysine = 1:50 at 22°C, and stir until the lysine is completely dissolved;
[0047] (3) Add benzyl chloroformate dropwise to the solution of step (2) in a molar ratio of lysine: benzyl chloroformate=1:50, while controlling the reaction temperature at 20 ± 1°C, and continue stirring 60 minutes;
[0048] (4) the reaction solution is adjusted to neutrality with hydrochloric acid;
[0049] (5) step (4) solution is extracted 5 times with ethyl acetate;
[0050](6) Combine the extracts, distill under reduced pressure at a temperature of 30° C. and a pressure of 25 mmHg to obtain the benzyloxycarbonyl pre-protected product of the α-amino group of lysine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com