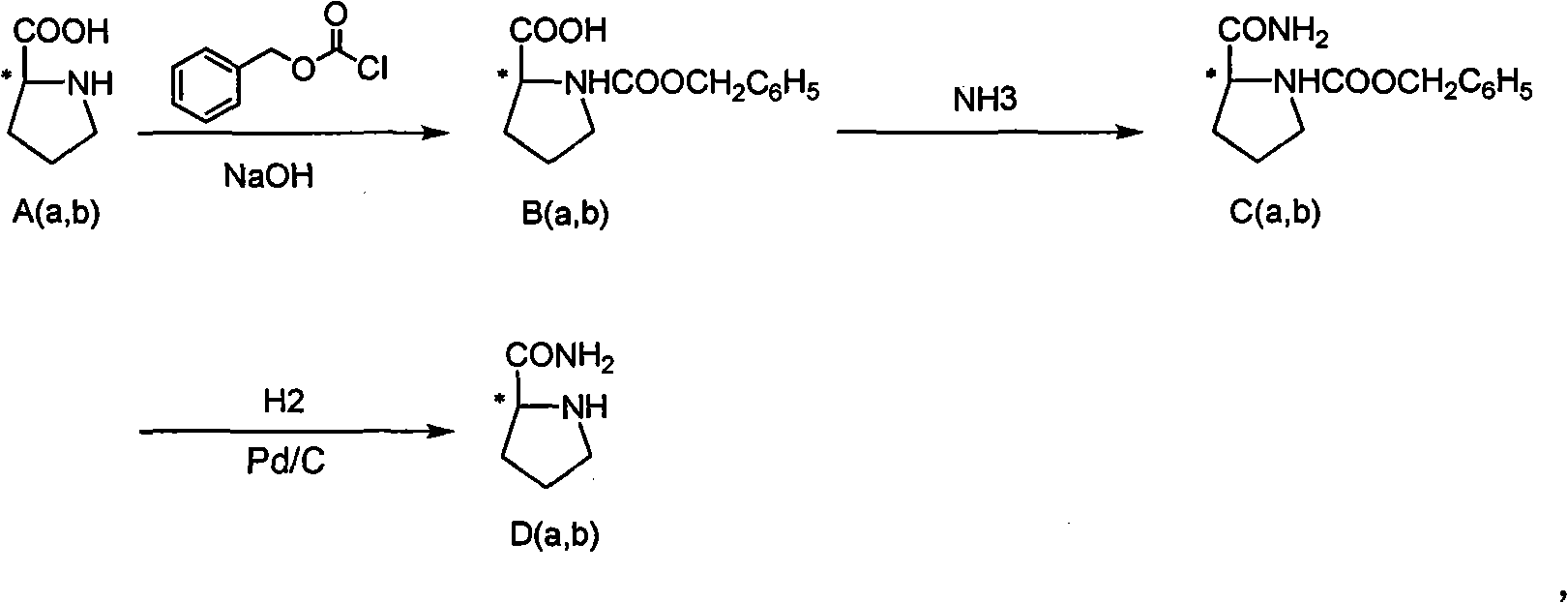
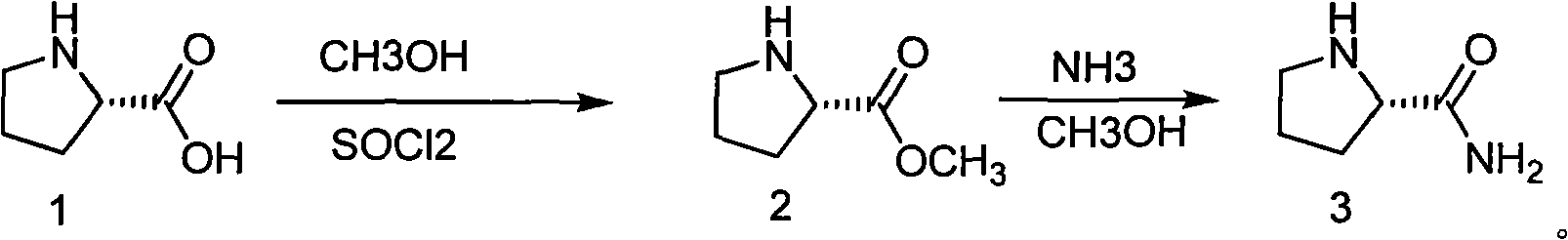
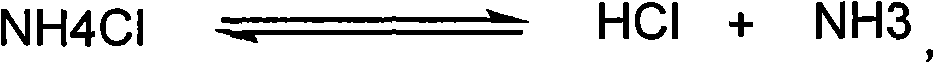Method for refining prolinamide
A technology of proline amide and proline ester, which is applied in the direction of organic chemistry, can solve the problems of product titration content decline, etc., and achieve the effects of not easy racemization, high optical purity and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of L-proline methyl ester hydrochloride.
[0030] Pump 500L of methanol into the reactor, and drop in 100 kg of L-proline. Stir and cool to 0~-10°C, and add 136 kg of thionyl chloride dropwise under temperature control. After dropping, the temperature was raised to reflux for 1 to 2 hours. Methanol was concentrated under reduced pressure to obtain a yellow oil.
Embodiment 2
[0031] Embodiment 2: Preparation of D-proline methyl ester hydrochloride.
[0032] Pump 500L of methanol into the reactor, and drop in 100 kg of D-proline. Stir and cool to 0~-10°C, and add 136 kg of thionyl chloride dropwise under temperature control. After dropping, the temperature was raised to reflux for 1 to 2 hours. Methanol was concentrated under reduced pressure to obtain a yellow oil.
Embodiment 3
[0033] Embodiment 3: Preparation of D-proline ethyl ester hydrochloride.
[0034] Pump 500L of ethanol into the reactor, and drop in 100 kg of D-proline. Stir and cool to 0~-10°C, and add 136 kg of thionyl chloride dropwise under temperature control. After dropping, the temperature was raised to reflux for 1 to 2 hours. Ethanol was concentrated under reduced pressure to obtain a yellow oil.
[0035] step 2
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com