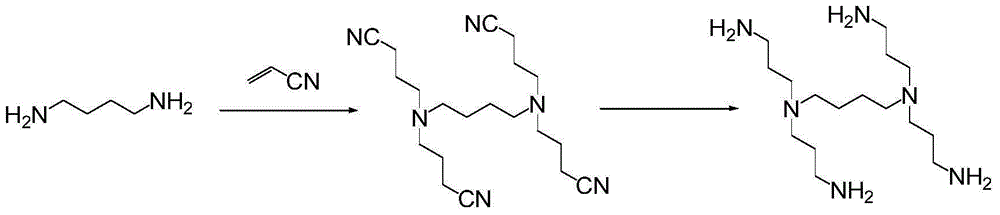
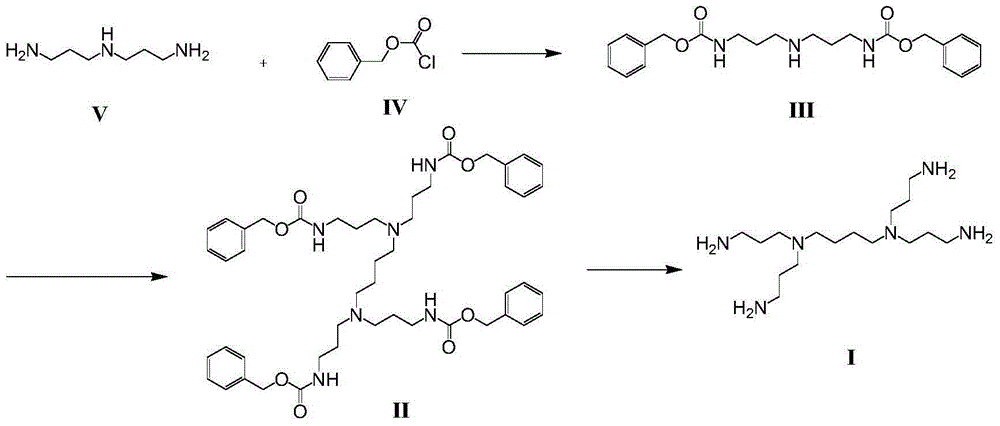
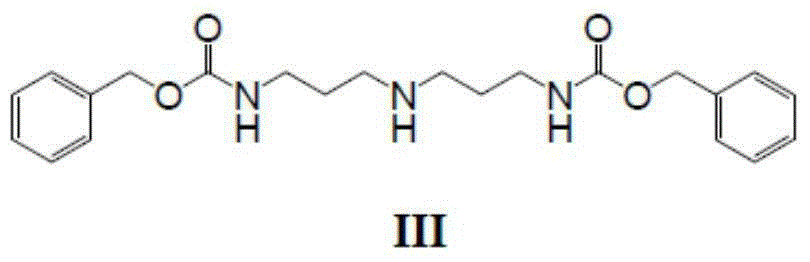
Bixalomer intermediate
A technology of pisalom and intermediates, which is applied in the field of amine phosphate binding agent pisalom intermediates and their preparation, can solve the problems of difficult complete reaction, danger, low yield and the like, and achieves simple reaction operation, three wastes and the like. The effect of less and higher yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of 3,3'-dibenzyloxyamide dipropylamine (compound III)
[0029] Add 4g of 3,3'-diaminodipropylamine, 9.3g of triethylamine and 30mL of dichloromethane into a 100mL reaction flask, control the temperature at 0-10°C, mix 15.5g of benzyl chloroformate and 20mL of dichloromethane Afterwards, it was added dropwise into the reaction flask, and the reaction was monitored by TLC to be complete. Pour the reaction solution into a beaker, stir and wash with 3*200mL saturated aqueous sodium carbonate solution, then wash with 200mL saturated brine, separate the layers, dry with anhydrous sodium sulfate, filter and spin off the solvent to obtain 11.46g3,3' -dibenzyloxyamide dipropylamine, the productive rate is 97.1%, and the HPLC detection purity is 94.2%. 2 g was purified by column chromatography to obtain 1.4 g of pure product.
[0030] MS(ESI+,m / z)(M+H) + = 400.26.
[0031] 13 C-NMR (300MHz, CDCl 3 ):δ=28.2(-CH 2 CH 2 CH 2 -),39.2(-CONHCH 2 -),46.6(-...
Embodiment 2
[0033] Example 2: Synthesis of 3,3'-dibenzyloxyamide dipropylamine (compound III)
[0034] Add 4g of 3,3'-diaminodipropylamine, 9.3g of triethylamine and 30mL of dichloromethane into a 100mL reaction flask, control the temperature at 0-10°C, mix 10.3g of benzyl chloroformate and 20mL of dichloromethane Afterwards, it was added dropwise into the reaction flask, and the reaction was monitored by TLC to be complete. Pour the reaction liquid into a beaker, stir and wash with 3*200mL saturated aqueous sodium carbonate solution, then wash with 200mL saturated brine, separate the layers, dry with anhydrous sodium sulfate, filter and spin off the solvent to obtain 10.9g3,3' -dibenzyloxyamide dipropylamine, the productive rate is 89.5%, and the HPLC detection purity is 98.9%.
[0035] MS(ESI+,m / z)(M+H) + = 400.26.
Embodiment 3
[0036] Example 3: Synthesis of 3,3'-dibenzyloxyamide dipropylamine (compound III)
[0037] Add 4g of 3,3'-diaminodipropylamine, 9.3g of triethylamine and 30mL of dichloromethane into a 100mL reaction flask, control the temperature at 0-10°C, mix 13.0g of benzyl chloroformate and 20mL of dichloromethane Afterwards, it was added dropwise into the reaction flask, and the reaction was monitored by TLC to be complete. Pour the reaction solution into a beaker, stir and wash with 3*200mL saturated aqueous sodium carbonate solution, then wash with 200mL saturated brine, separate the layers, dry with anhydrous sodium sulfate, filter and spin off the solvent to obtain 11.6g3,3' -dibenzyloxyamide dipropylamine, the yield is 95.3%, and the HPLC detection purity is 97.6%.
[0038] MS(ESI+,m / z)(M+H) + = 400.26.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com