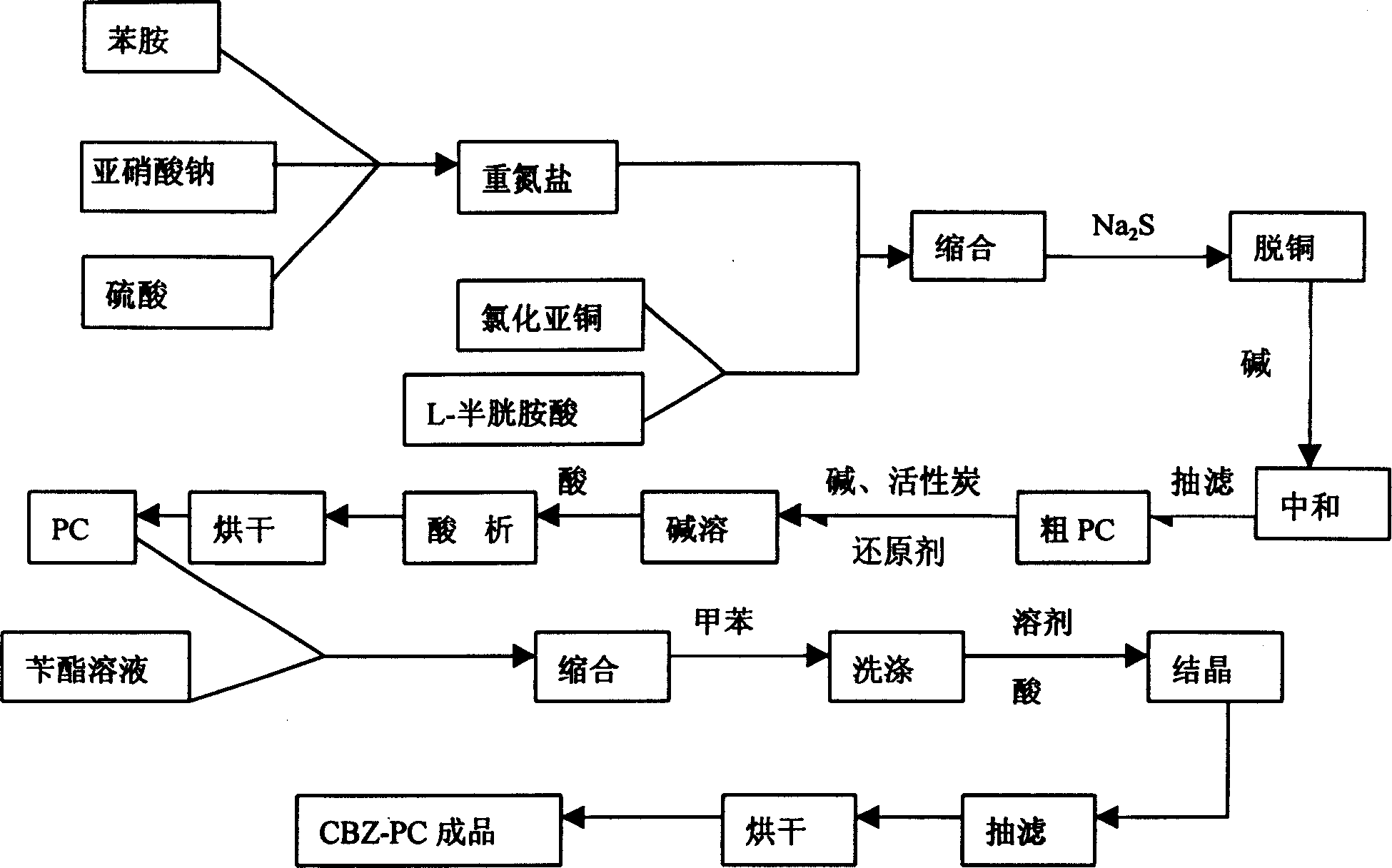
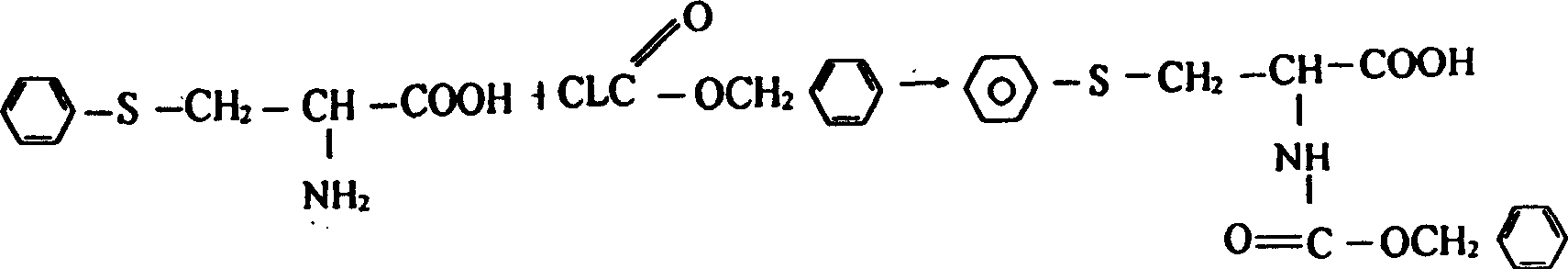Technique for preparing N-carbobenzoxy-5 phenyl-L-cysteine
The technology of cysteine and benzyloxycarbonyl is applied in the field of preparation technology of N-benzyloxycarbonyl-S-phenyl-L-cysteine, which can solve the problem of low quality of CBZ-PC products and high price of serine raw materials , can not meet user requirements and other problems, to achieve the effect of reduced production cost, good quality and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 52g (28ml) of sulfuric acid in 275ml of water, add 22.5g of aniline solution, cool to below 5°C, dissolve 8.75g of sodium nitrite in 40ml of water, and add dropwise to the above solution to obtain aniline diazonium salt;
[0021] Dissolve 30g of L-cysteine hydrochloride (0.17mol) containing one crystalline water in 225g of water, add 11g of 30% industrial hydrochloric acid, then add 4.25g of cuprous chloride, cool to below 5°C, and prepare the above Add the obtained diazonium salt into the solution dropwise, continue to react at this temperature for 6 hours after dropping, then raise the temperature of the reactant to 85°C-90°C, keep it warm for 1 hour, then cool to about 60°C and add sodium sulfide aqueous solution Suction filtration until no precipitate is formed, the solution is neutralized to PH5-7 with 30% sodium hydroxide, suction filtration, crude PC is dissolved in sodium hydroxide solution, 5g of activated carbon and reducing agent are added for decol...
Embodiment 2
[0024] Other methods are the same as in Example 1, except that 100 ml of toluene is added to the toluene-washed solution prepared in the same way as in Example 1, then acidified to PH2 with hydrochloric acid, stirred and crystallized at room temperature, and dried by suction to obtain 9.0 g of CBZ-PC , the content is 99.30%, the melting point is 93.5-94.5°C, and the yield is 89%.
Embodiment 3
[0026] Other methods are the same as in Example 1, except that the solution after washing with toluene obtained in the same way as in Example 1 is added with 5ml of carbon tetrachloride and 40ml of ethylene dichloride, acidified to PH2, cooled to 5 ° C, stirred and crystallized , filtered and dried to obtain 7.4g of CBZ-PC, with a content of 99.61%, a melting point of 94-95.5°C, and a yield of 73.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com