Synthesis method of lifitegrast intermediate
A technology of rifemilast and synthetic method, which is applied in the field of chemical pharmacy, can solve problems such as unstable process, slow reaction speed, difficult post-processing, etc., and achieve the effects of strong process controllability, reduced amount of impurities, and widened range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
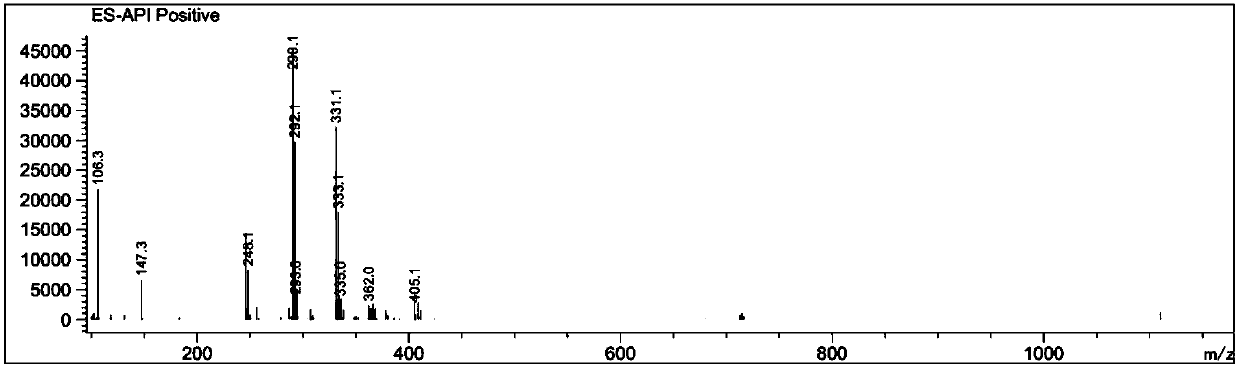
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of rifemilast intermediate of the present invention
[0041] (1) Synthesis of benzyl 5,7-dichloro-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinoline-6-carboxylate
[0042] In a 5L reactor, under mechanical stirring, sequentially add 1.20kg of tetrahydrofuran, 200g of 5,7-dichloro-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinoline and N,N,N , N-tetramethylethylenediamine 100g, nitrogen gas was passed into the reaction kettle for 15min to exhaust the air. Cool down the reactor system to -75~-78°C. Add 300ml of 2.5M n-butyllithium solution dropwise and control the rate of addition to maintain the internal temperature at -70°C to -78°C. After the dropwise addition, keep at -70°C to -78°C for 1h. Add 120g of benzyl chloroformate dropwise, control the feed rate to keep the internal temperature not exceeding -70°C, monitor the reaction by HPLC method, after the reaction is complete, add saturated ammonium chloride water to the reaction syst...
Embodiment 2
[0053] Embodiment 2, the preparation of rifemilast intermediate of the present invention
[0054] (1) Synthesis of benzyl 5,7-dichloro-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinoline-6-carboxylate
[0055] In a 10L reactor, under mechanical stirring, 2.6kg tetrahydrofuran, 400g 5,7-dichloro-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinoline and 240g N,N, N,N-Tetramethylethylenediamine, blow nitrogen into the reaction kettle for 20 minutes to exhaust the air. Cool down the reactor system to -75~-78°C. Add 600ml of 2.5M n-butyllithium solution dropwise and control the rate of addition to keep the internal temperature at -70°C to -78°C. After the dropwise addition, keep at -70°C to -78°C for reaction. 250 g of benzyl chloroformate was added dropwise, and the feed rate was controlled to keep the internal temperature not exceeding -70°C. The reaction was monitored by HPLC. After the reaction was complete, add saturated ammonium chloride water to the reaction system to qu...
Embodiment 3
[0060] Embodiment 3, the preparation of rifemilast intermediate of the present invention
[0061] (1) Synthesis of benzyl 5,7-dichloro-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinoline-6-carboxylate
[0062] In a 50L reactor, under mechanical stirring, 11.8kg of tetrahydrofuran, 1.70kg of 5,7-dichloro-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinoline and N,N, N,N-Tetramethylethylenediamine 0.85kg, feed nitrogen into the reaction kettle for 25min to exhaust the air. Cool down the reactor system to -75~-78°C. Add 2.71 L of 2.5M n-butyllithium solution dropwise and control the rate of addition to maintain the internal temperature at -70°C to -78°C. After the dropwise addition is complete, keep the reaction below -70°C to -78°C. Add 1.16 kg of benzyl chloroformate, and control the feed rate to keep the internal temperature not exceeding -70°C. The reaction is monitored by HPLC method. After the reaction is complete, add saturated ammonium chloride water to the reaction s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com