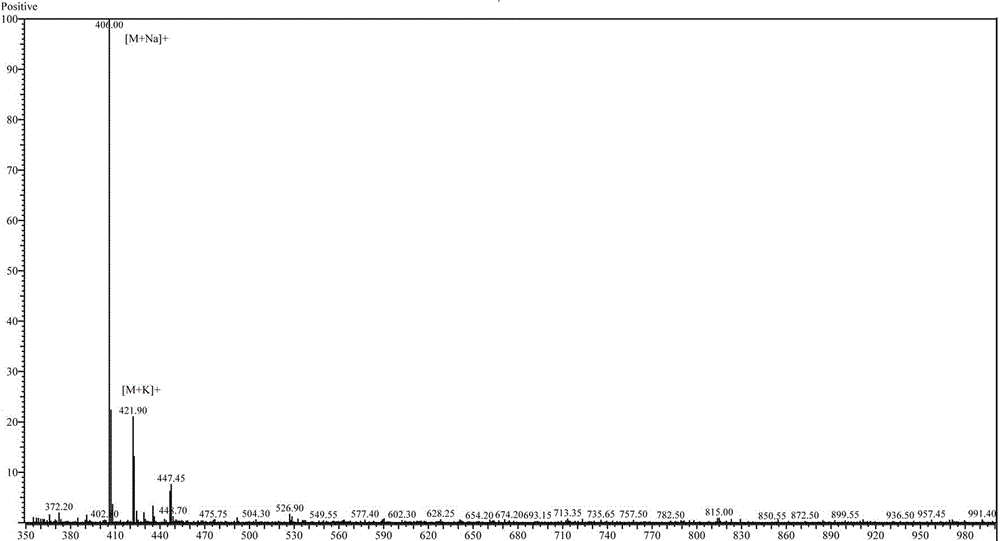
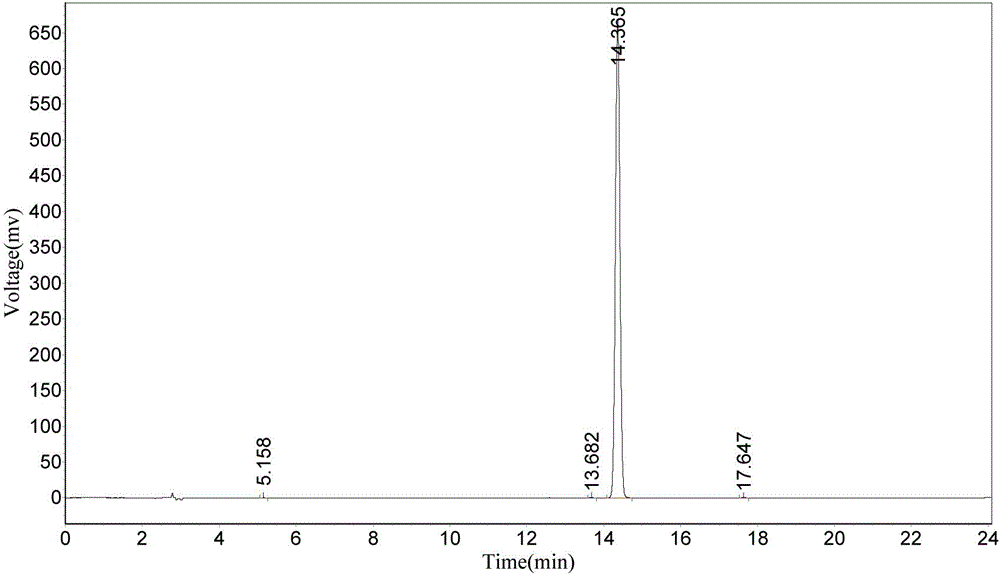
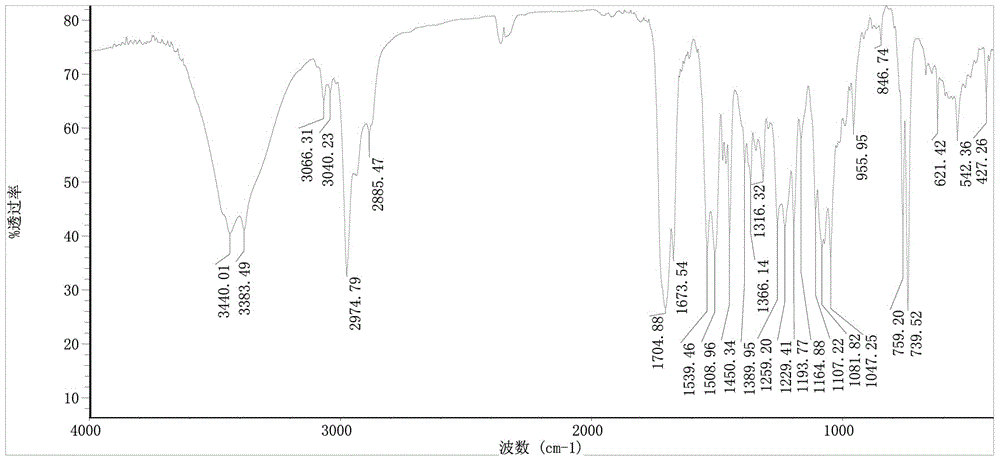
Synthesis method of Fmoc-O-tert-butyl-L-threoninol
A synthetic method and technology of threonine alcohol, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbamic acid derivatives, etc., can solve the problems of high risk, high cost, low yield, etc., and achieve increased yield , low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0023] 1. Add 70g of L-threonine and 500ml of methanol to a 1000ml three-neck flask, control the temperature below 0°C, and add 64ml of thionyl chloride dropwise. After the dropwise addition, the mixture was heated to reflux for 4 hours. Distilled to dryness under reduced pressure at 55°C to obtain 100 g of L-threonine methyl ester hydrochloride oil. Calculate next step reaction with productive rate 100%;
[0024] 2. In a 2L beaker, add 100g of L-threonine methyl ester hydrochloride, 600ml of water, adjust the pH to alkaline with sodium bicarbonate, add 110 g of z-cl dropwise and adjust it with 2moc / l sodium hydroxide The pH reacts at 8-9. After the reaction, add 1L of ethyl acetate for extraction, wash with 500ml of water, wash with 500ml of saturated brine, dry with 100g of anhydrous sodium sulfate, filter with suction, distill under reduced pressure until solid precipitates, add 600Ml of petroleum ether for crystallization, filter with suction, and dry to obtain z-thr -o...
experiment example 2
[0030] Experimental example 2: 1-4 steps are the same and will not be repeated.
[0031] 5. Put 130g of z-thr(tbu)-oh into a 2L three-necked flask, add 1300ml of tetrahydrofuran, T<0°C, add dropwise 42.5g of N-methylmorpholine, 45.6g of ethyl chloroformate, 15.8g of sodium borohydride g / 139ml (3mol / l) water. After reacting overnight, add ethyl acetate 800Ml for extraction. Wash with 300ml of water, 300ml of saturated brine, and dry with 100g of anhydrous sodium sulfate. Suction filtration, concentrated under reduced pressure to obtain 96g of product, yield 78.5%;
[0032] 6. Add 96g of Z-thr(tbu)-o to a 1L three-necked flask, add 800ML of methanol, 8.3g of 5% palladium carbon, and pass through hydrogen to react. After the reaction is completed, filter with suction and concentrate to dryness under reduced pressure to obtain H-thr( tbu)-ol 54g, calculates with the next reaction of productive rate 100%;
[0033] 7. In a 2L beaker, add 54g of H-thr(tbu)-ol, 300ml of water, 500...
experiment example 3
[0034] Experimental Example 3: Steps 1-4 are the same and will not be repeated
[0035] 5. Put 130g of z-thr(tbu)-oh into a 2L three-necked flask, add 1300ml of tetrahydrofuran, T<0°C, add dropwise 42.5g of N-methylmorpholine, 45.6g of ethyl chloroformate, 15.8g of sodium borohydride g / 83.5ml (5mol / l) water. After reacting overnight, add ethyl acetate 800Ml for extraction. Wash with 300ml of water, 300ml of saturated brine, and dry with 100g of anhydrous sodium sulfate. Suction filtration, concentrated under reduced pressure to obtain product 92g yield 74.7%;
[0036] 6. Add 92g of Z-thr(tbu)-o to a 1L three-necked flask, add 800ML of methanol, 8.3g of 5% palladium carbon, and pass through hydrogen to react. After the reaction is completed, filter with suction, and concentrate to dryness under reduced pressure to obtain H-thr( tbu)-ol 53g is calculated with the next step reaction of productive rate 100%;
[0037] 7. In a 2L beaker, add H-thr(tbu)-ol 53g, water 300ml, aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com