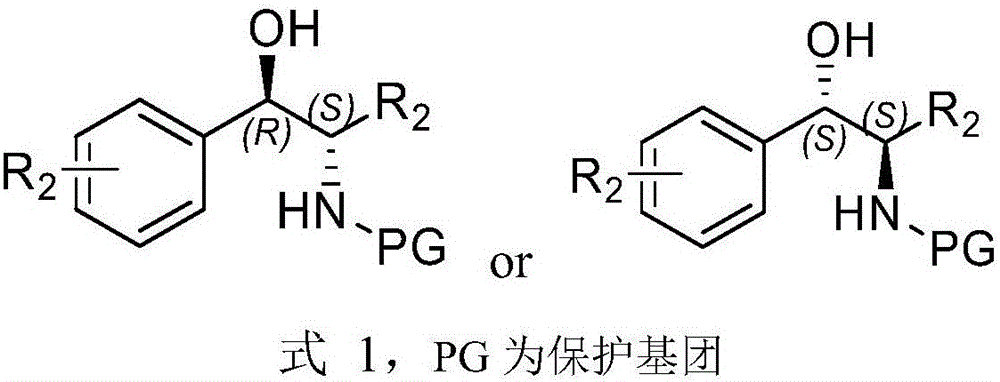
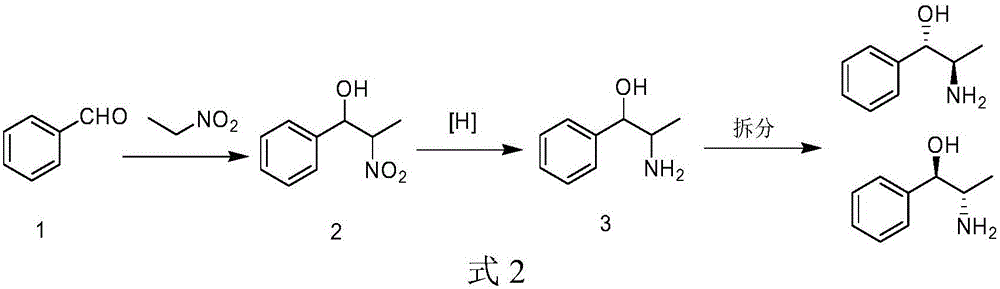
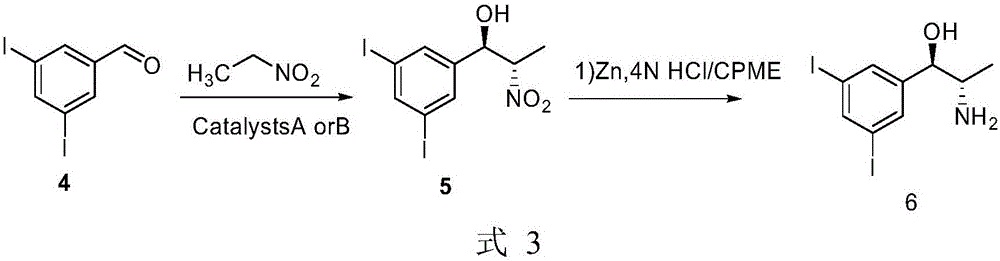
Method for preparing arene beta-amino alcohol of optical voidness
A technology for amino alcohols and aromatic hydrocarbons, applied in the field of preparation of aromatic hydrocarbon β-amino alcohols, can solve the problems of unsuitability for long-term storage, increased production cost, easy racemization of compound 4, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Synthesis of key intermediates of alogliptin
[0113] 1. Preparation of (4S)-N-Boc-4-propargyl-5-oxazolidinone, namely the compound of formula 22
[0114]
[0115] With 19.9g, 0.1mol (L)-N-BOC-propargylglycine, 6.7g paraformaldehyde and 0.19g, 1mmol p-toluenesulfonic acid monohydrate in 190ml toluene, the reaction solution was suspended; heated to In the reflux state, the produced water is separated by a water separator, and the reaction liquid gradually becomes clear as the reaction progresses. After the reaction was completed, the reaction liquid was cooled to room temperature, an aqueous solution of sodium bicarbonate was added to the reaction liquid for washing, the layers were separated, and the organic layer was washed with saturated brine. The toluene was distilled off under reduced pressure, and the residue was crystallized by cooling and filtered to obtain compound 22, 18.8 g of white crystals, with a yield of 89%.
[0116] 1 H-NMR (CD 3 Cl, 400MHz) δppm...
Embodiment 2
[0138] Synthesis of key intermediates of alogliptin
[0139] 1. Preparation of (4S)-N-Boc-4-propargyl-5-oxazolidinone, namely the compound of formula 22
[0140]
[0141] With 19.9g, 0.1mol of (L)-N-BOC-propargylglycine, 39.8g of paraformaldehyde and 0.38g, 2mmol of p-toluenesulfonic acid monohydrate in 250ml of toluene, the reaction solution was suspended; heating To the state of reflux, use the water separator to divide the produced water. As the reaction progresses, the reaction solution gradually becomes clear. After the reaction was completed, the reaction liquid was cooled to room temperature, an aqueous solution of sodium bicarbonate was added to the reaction liquid for washing, the layers were separated, and the organic layer was washed with saturated brine. Toluene was distilled off under reduced pressure, and the residue was crystallized by cooling and filtered to obtain the title compound 22, 20 g of white crystals, with a yield of 95%.
[0142] 2. Preparation ...
Embodiment 3
[0152] Synthesis of key intermediates of ansertrapib
[0153] 1, the preparation of (4S)-N-benzyloxycarbonyl-4-methyl-5-oxazolidinone, namely the compound of formula 24
[0154]
[0155] Put 22.3g, 0.1mol of benzyloxycarbonyl-L-alanine, 8.2g of paraformaldehyde and 0.19g, 1mmol of p-toluenesulfonic acid monohydrate in 220ml of toluene, the reaction solution was suspended; heated to reflux state, use a water separator to divide the produced water, and as the reaction progresses, the reaction solution gradually becomes clear. After the reaction was completed, the reaction solution was cooled to room temperature, 50 ml of aqueous sodium bicarbonate solution was added to the reaction solution for washing, the layers were separated, and the organic layer was washed with saturated brine. The toluene was distilled off under reduced pressure, and the residue was crystallized by cooling and filtered to obtain the title compound 22, 21.6 g of white crystals, with a yield of 92%. Me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com