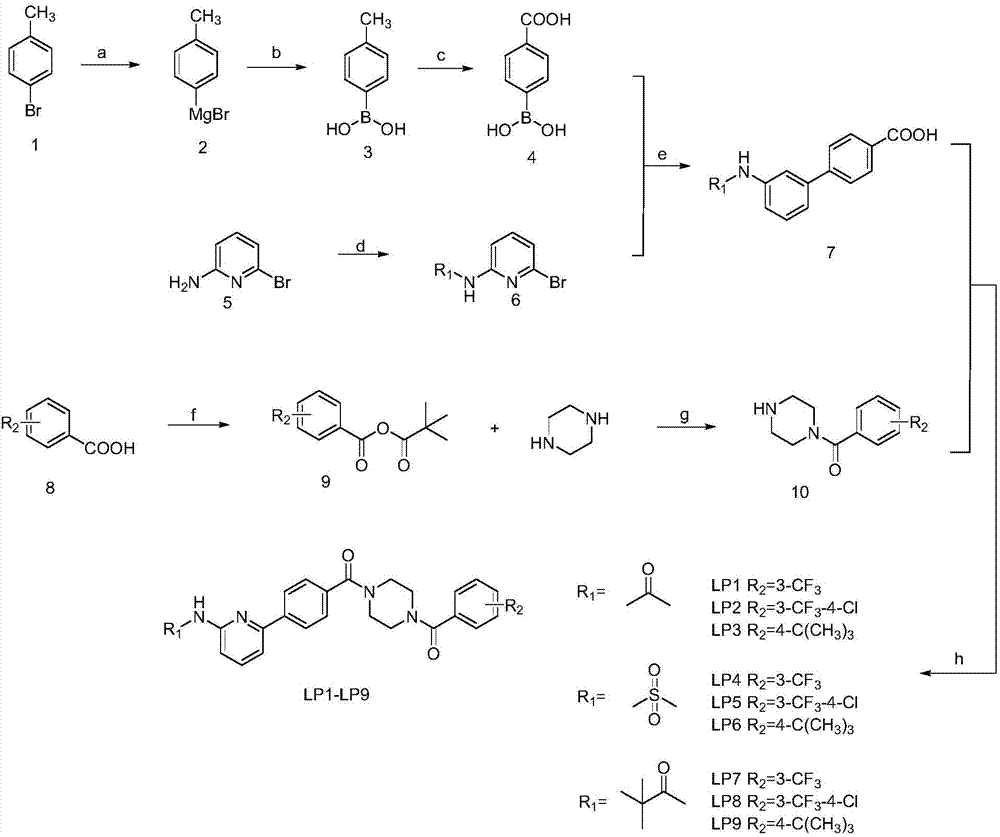
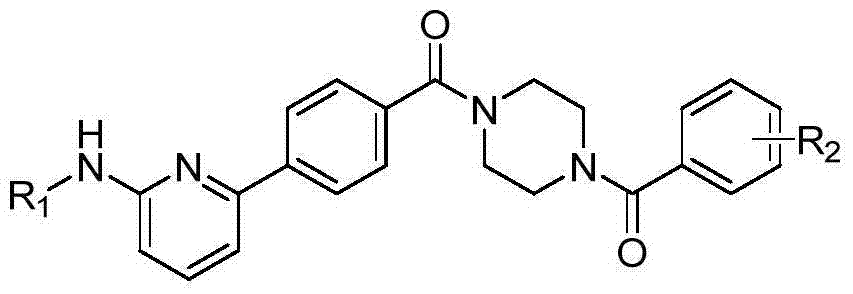
6-phenylpyridine-2-amine Bcr-Abl inhibitors as well as preparation method and application thereof
A technology of bcr-abl and phenylpyridine, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as drug resistance and mutation resistance, and achieve cheap reagents and easy availability of raw materials. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In the structural formula of the inhibitor, R 1 is an acetylated group, R 2 For m-trifluoromethyl, prepared by the following steps:
[0043] 1) Preparation of p-carboxyphenylboronic acid (compound 4)
[0044] Add treated Mg strips (2.15g, 90mmol), 2 capsules of iodine into a 250ml double-necked bottle, protect with nitrogen, and vacuumize 3 times. Slowly add the anhydrous THF solution of the compound p-methylbromobenzene (compound 1, 60mmol) with a syringe under heating conditions. After the reaction is initiated, it is in a reflux state. Continue to add the remaining solution. After the addition, reflux for 5 hours to obtain the format of p-bromotoluene Reagent (compound 2), after cooling to room temperature, the reaction device was transferred to a low-temperature reactor, and the temperature was adjusted to -20 ° C. After 5 minutes, anhydrous THF solution of trimethyl borate (9.36 g, 90 mmol) was added with a syringe, After the addition, react at room temperature ...
Embodiment 2
[0060] In the structural formula of the inhibitor, R 1 is a mesylation group, R 2 is 4-chloro-3-trifluoromethyl, prepared by the following steps:
[0061] Step 1) is the same as Step 1) in Example 1, that is, p-carboxyphenylboronic acid (compound 4) is prepared from p-bromotoluene (compound 1).
[0062] 2) Preparation of N-(6-bromopyridin-2-yl)methanesulfonamide (compound 6)
[0063] 2-Amino-6-bromopyridine (compound 5, 5.19 g, 30 mmol) was dissolved in 100 ml of anhydrous dichloromethane, 20 ml of anhydrous triethylamine was added, and stirred in an ice bath for 30 minutes. A solution of methanesulfonyl chloride (60mmol, 4.64ml) in anhydrous dichloromethane (40ml) was added dropwise in an ice bath. After the dropwise addition, the ice bath was removed, and the mixture was raised to room temperature to react overnight. After the reaction, dilute with dichloromethane, wash with water (30ml×3), wash with sodium bicarbonate solution (30ml×3), wash with saturated sodium chlori...
Embodiment 3
[0077] In the structural formula of the inhibitor, R 1 is a pivaloyl group, R 2 For p-tert-butyl, prepared by the following steps:
[0078] Step 1) is the same as Step 1) in Example 1, that is, p-carboxyphenylboronic acid (compound 4) is prepared from p-bromotoluene (compound 1).
[0079] 2) Preparation of N-(6-bromopyridin-2-yl)-2,2-dimethylpropionamide (compound 6)
[0080] 2-Amino-6-bromopyridine (compound 5, 5.19 g, 30 mmol) was dissolved in 100 ml of anhydrous dichloromethane, 20 ml of anhydrous triethylamine was added, and stirred in an ice bath for 30 minutes. A solution of pivaloyl chloride (60mmol, 7.39ml) in anhydrous dichloromethane (40ml) was added dropwise under ice-cooling conditions. After the dropwise addition, the ice bath was removed, and the mixture was raised to room temperature to react overnight. After the reaction, dilute with dichloromethane, wash with water (30ml×3), wash with sodium bicarbonate solution (30ml×3), wash with saturated sodium chlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com