N,6 diphenylpyrimidine-4-amine Bcr-Abl inhibitors as well as preparation method and application thereof
A technology of diphenylpyrimidine and bcr-abl, which is applied in the field of biomedicine, can solve problems such as drug resistance and mutation resistance, and achieve the effect of cheap reagents, easy-to-obtain raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
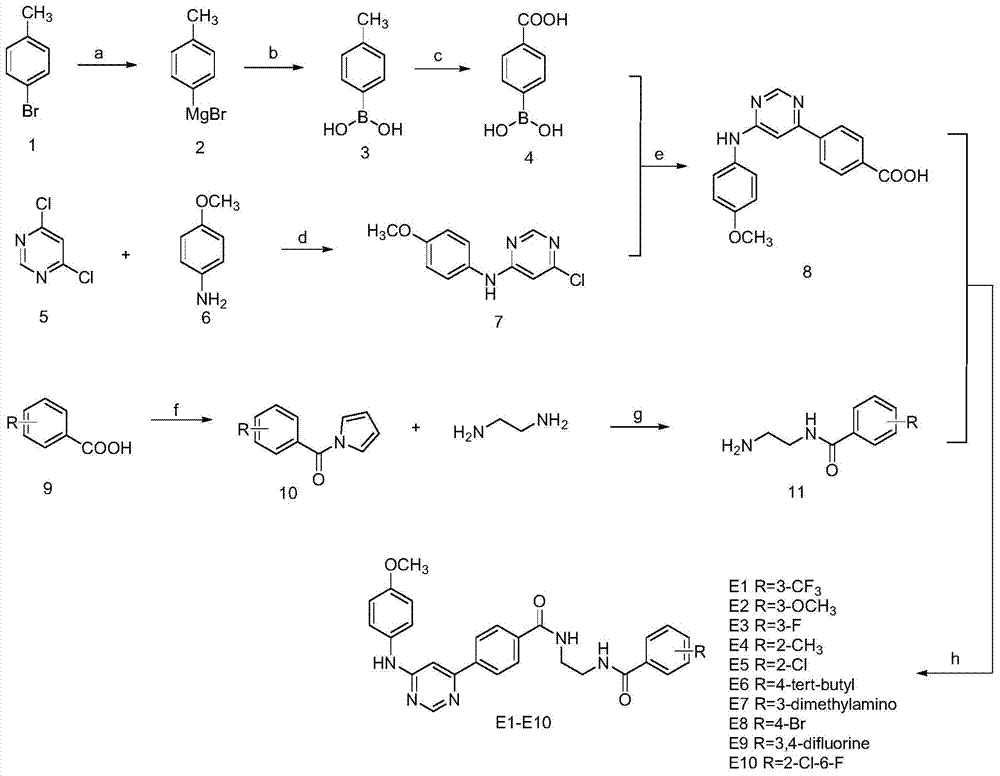
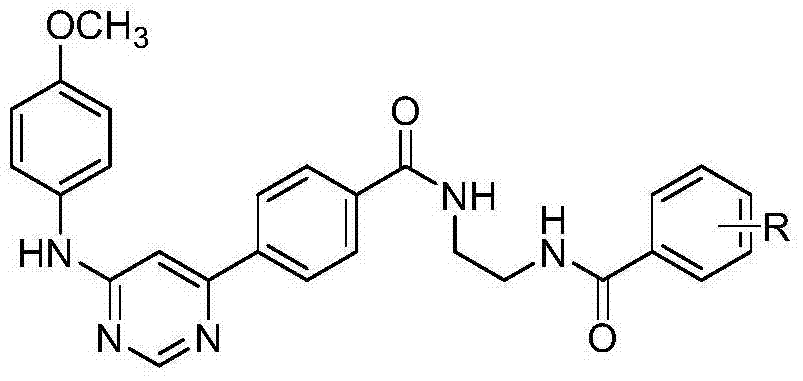
[0041] In the structural formula of the inhibitor, the R group is m-trifluoromethyl, which is prepared by the following steps:
[0042] 1) Preparation of p-carboxyphenylboronic acid (compound 4)
[0043] Add treated Mg strips (2.15g, 90mmol), 2 capsules of iodine into a 250ml double-necked bottle, protect with nitrogen, and vacuumize 3 times. Slowly add the anhydrous THF solution of the compound p-toluene (compound 1, 60mmol) with a syringe under heating conditions. After the reaction is triggered, it is in a reflux state, and the remaining solution is continued to be added. After the addition, the reaction is refluxed for 5 hours to obtain p-bromotoluene. Grignard reagent (compound 2), after cooling to room temperature, transfer the reaction device to a low-temperature reactor, adjust the temperature to -20°C, add trimethyl borate (9.36g, 90mmol) anhydrous THF solution with a syringe after 5 minutes , reacted at room temperature for 3 hours after the addition was completed, ...
Embodiment 2
[0059] In the structural formula of the inhibitor, wherein the R group is o-chloro, prepared by the following steps
[0060] Steps 1) to 3) are the same as steps 1) to 3) in Example 1, that is, p-carboxyphenylboronic acid (compound 4), 4,6-dichloropyrimidine and p-methoxy Aniline undergoes an electrophilic substitution reaction to give 6-chloro-N-(4-methoxyphenyl)pyrimidine 4-amine (compound 7), 6-chloro-N-(4-methoxyphenyl)pyrimidine 4-amine (Compound 7) and p-carboxyphenylboronic acid (Compound 4) were coupled by SUZUKI to obtain 4-{6-[(4-methoxyphenyl)amino]pyrimidin4-yl}benzoic acid (Compound 8).
[0061] 4) Condensation of o-chlorobenzoic acid (compound 9) and ethylenediamine by CDI to give N-(2-aminoethyl)-2-chlorobenzamide (compound 11)
[0062] Add o-chlorobenzoic acid (compound 9, 10mmol) and N,N'-carbonyldiimidazole (1.94g, 12mmol) into a 100ml beaker, and mechanically stir until the reaction system forms a viscous substance, that is, an active intermediate (compound...
Embodiment 3
[0071] In the structural formula of the inhibitor, the R group is a p-tert-butyl group, which is prepared by the following steps
[0072] Steps 1) to 3) are the same as steps 1) to 3) in Example 1, that is, p-carboxyphenylboronic acid (compound 4), 4,6-dichloropyrimidine and p-methoxy Aniline undergoes an electrophilic substitution reaction to give 6-chloro-N-(4-methoxyphenyl)pyrimidine 4-amine (compound 7), 6-chloro-N-(4-methoxyphenyl)pyrimidine 4-amine (Compound 7) and p-carboxyphenylboronic acid (Compound 4) were coupled by SUZUKI to obtain 4-{6-[(4-methoxyphenyl)amino]pyrimidin4-yl}benzoic acid (Compound 8).
[0073] 4) Condensation of p-tert-butylbenzoic acid (compound 9) and ethylenediamine by CDI gives N-(2-aminoethyl)-4-tert-butylbenzamide (compound 11)
[0074] Add p-tert-butylbenzoic acid (compound 9, 10mmol), N,N'-carbonyldiimidazole (1.94g, 12mmol) into a 100ml beaker, and mechanically stir until the reaction system forms a viscous substance, that is, an active in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com