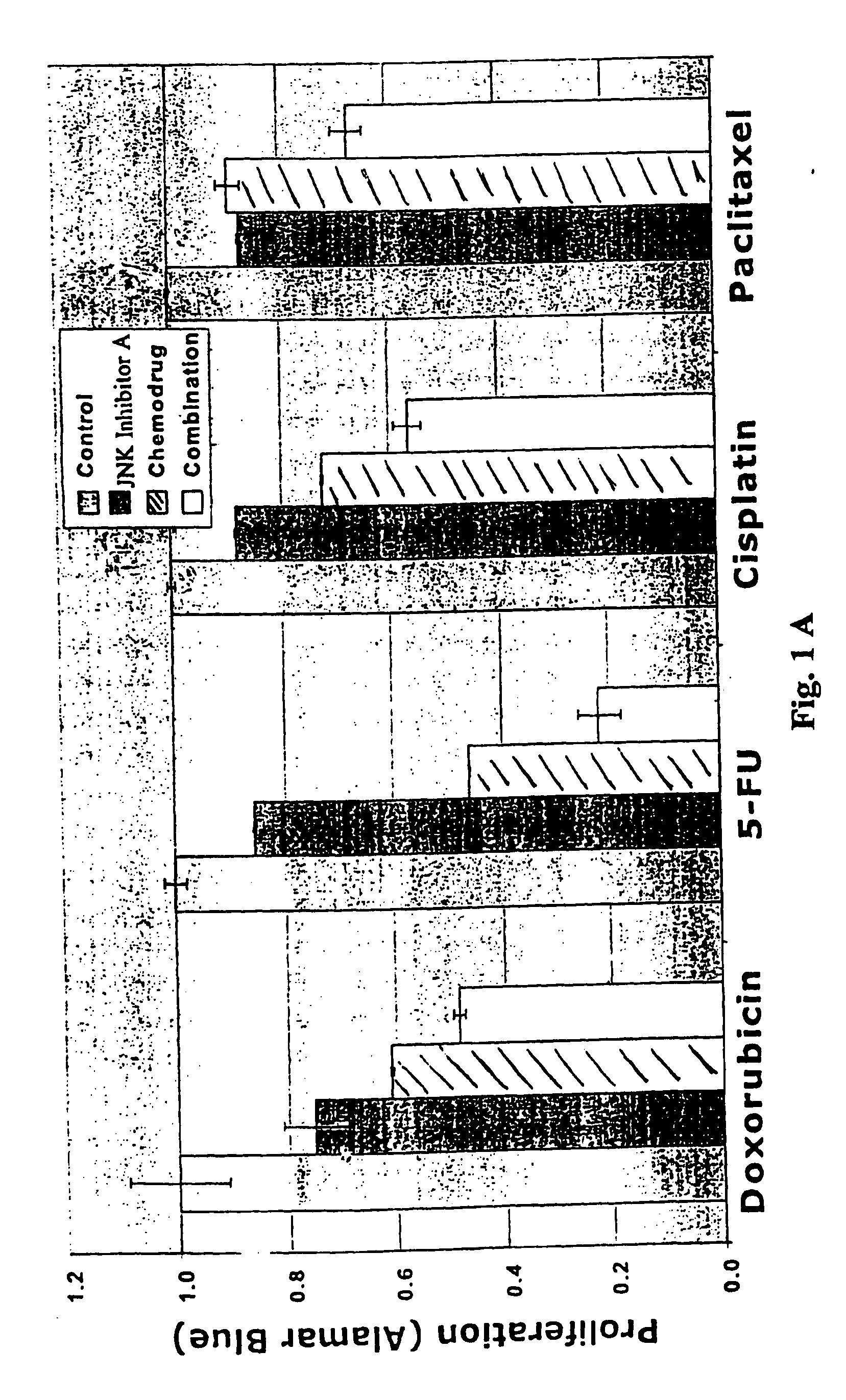
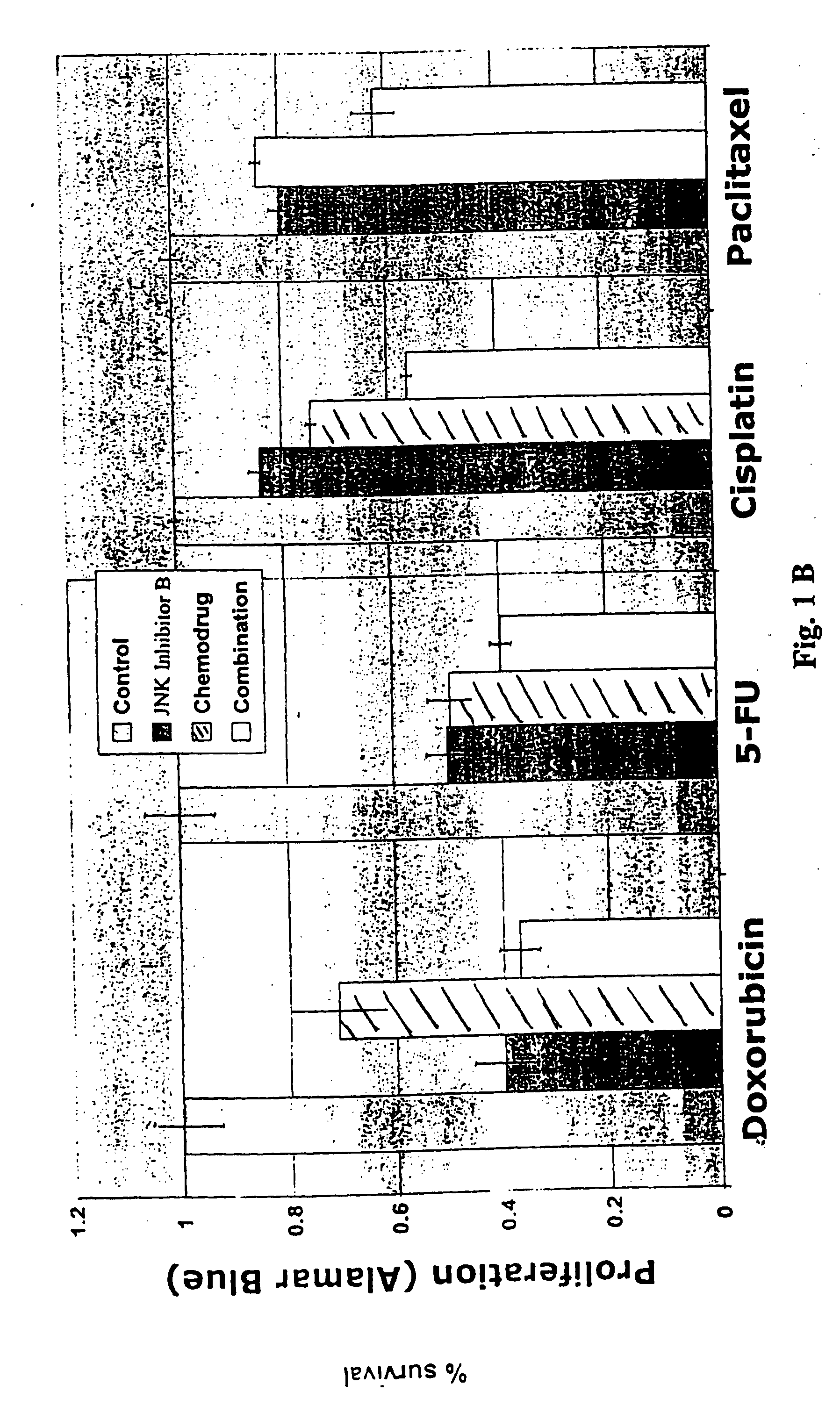
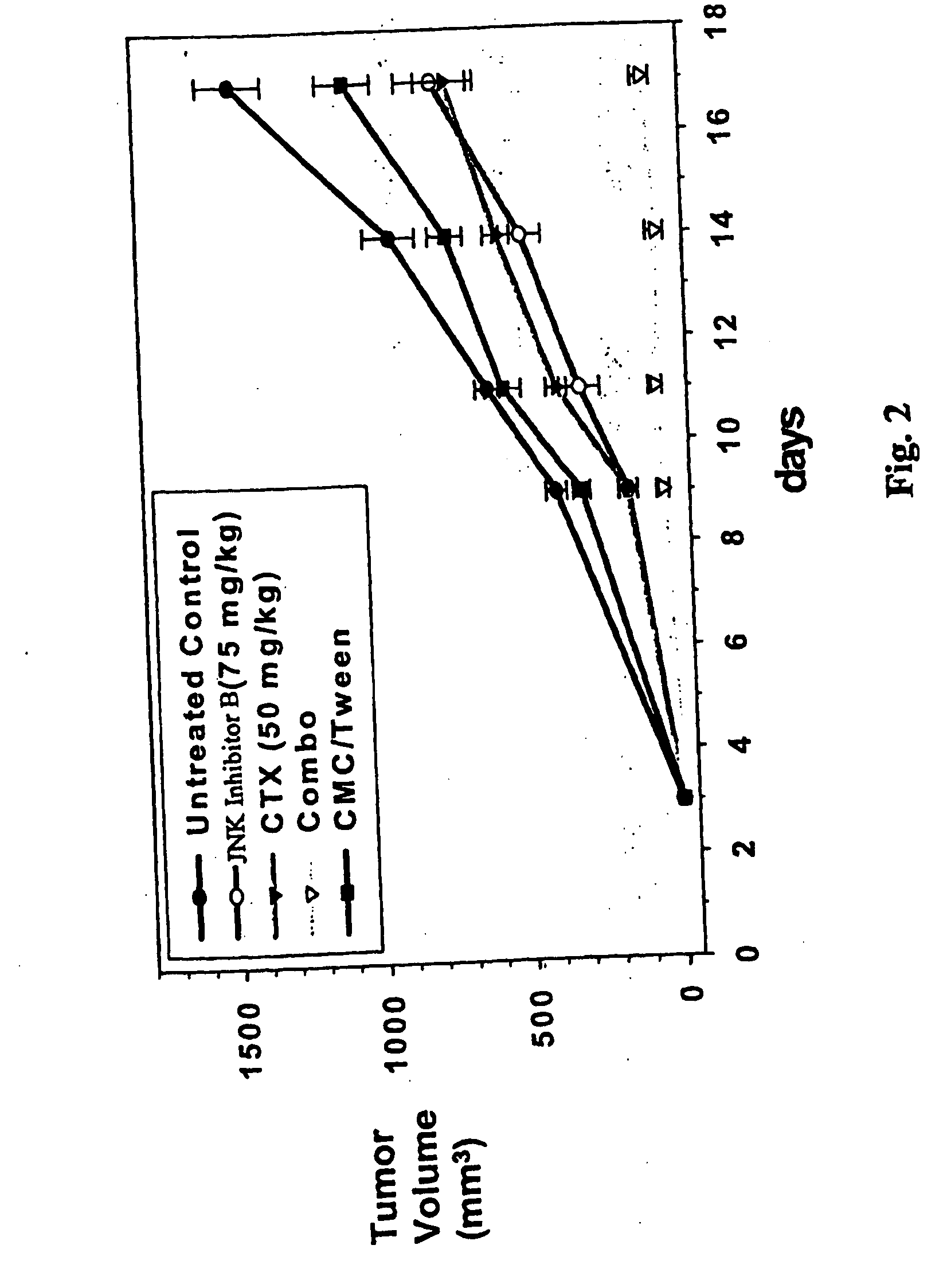
Combination therapy for treating or managing acute myelocytic leukemia
a myelocytic leukemia and therapy technology, applied in the field of combination therapies, can solve the problems of affecting the quality of life, huge burden on the health care industry, and jnk inhibitors may block transformation and tumor cell growth, so as to improve the tolerance, reduce side effects, and enhance the effect of effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0102] As mentioned above, the present invention is directed to methods useful for treating, preventing or managing cancer by administering to a patient in need thereof one or more JNK inhibitors in combination with one or more anti-cancer agents and / or radiation therapy. Representative JNK inhibitors of the present invention include, but are not limited to, indazoles, anilinopyrimidine, isothiazoloanthrones, isoxazoloanthrones, isoindolanthrones, pyrazoloanthrones and derivatives thereof.
[0103] In certain embodiments, inhibitors of JNK decrease the activity of JNK. In other embodiments, inhibitors of JNK decrease the amount of JNK present in the cell. In other embodiments, inhibitors of JNK decrease the amount of JNK mRNA, or mRNA of another component of the JNK pathway, in the cell.
[0104] In one embodiment, the inhibitor of JNK is a small organic molecule capable of inhibiting JNK activity or another component of the JNK pathway. In another embodiment, the inhibitor of JNK is an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com