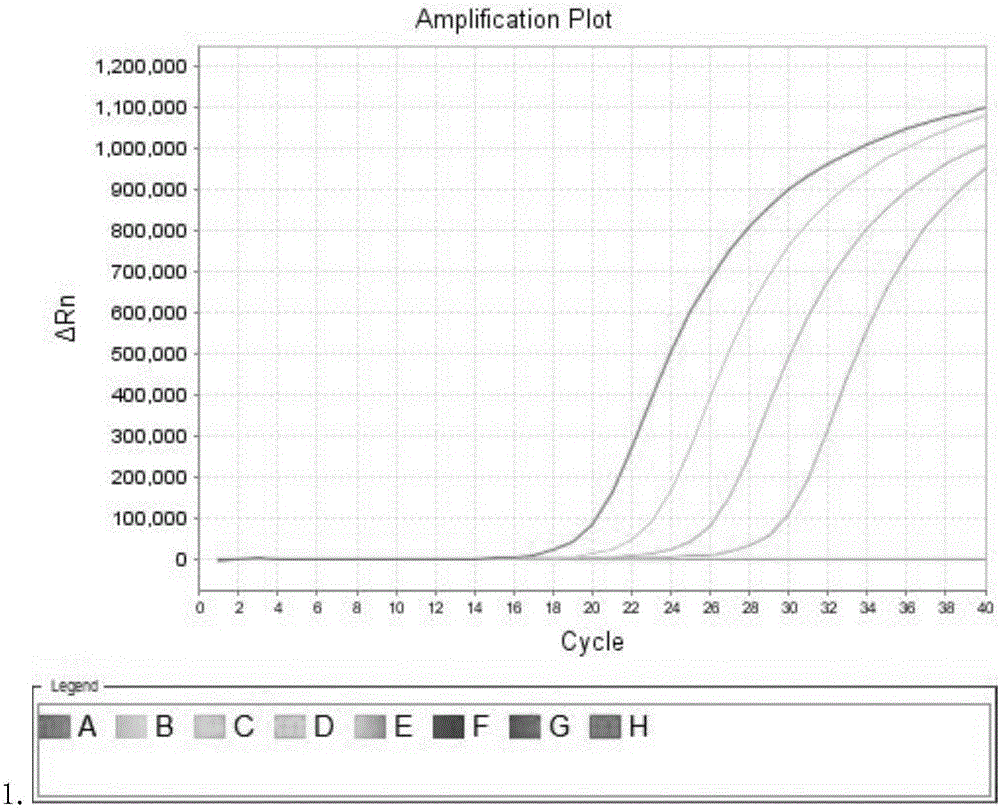
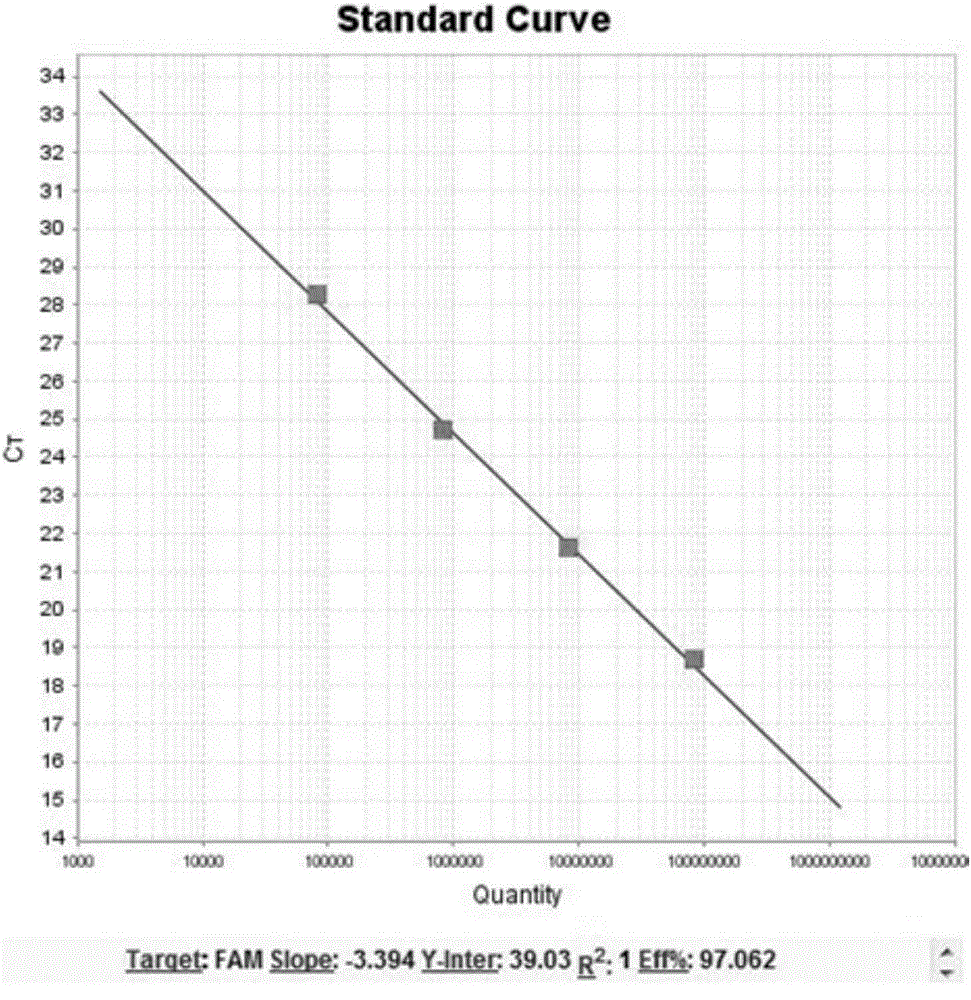
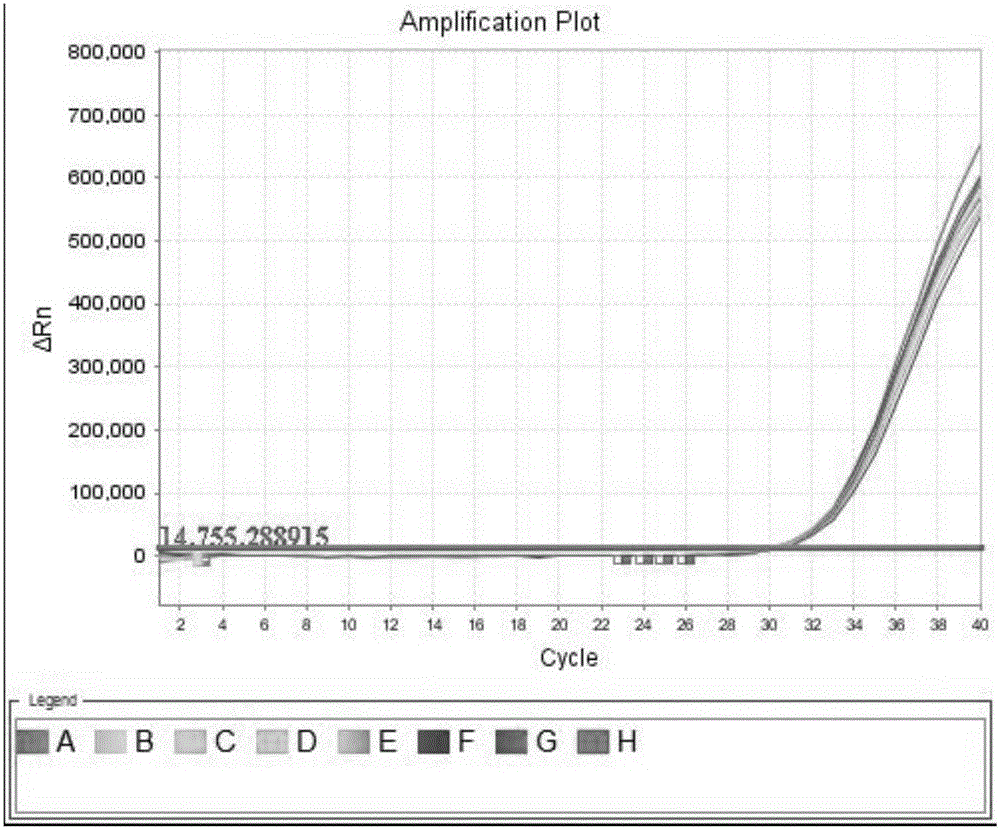
BCR-ABL fusion gene amplification kit and BCR-ABL fusion gene detection kit
A detection kit and gene fusion technology, which is applied in the field of gene amplification, can solve the problems of increased detection time, false positives, and easy failure of culture, and achieve the effects of reducing RNA loss, preventing PCR pollution, and reducing false positive results
Inactive Publication Date: 2017-02-15
SHANGHAI REPODX BIOTECH CO LTD
View PDF6 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0015] 3. With the continuous development of detection technology, the detection method of BCR-ABL fusion gene is also constantly progressing, from traditional cytogenetics method to fluorescence in situ hybridization method, to molecular level polymerase chain reaction, These methods can detect BCR-ABL transcripts, but various detection methods have their advantages and disadvantages in sample processing and detection operations. In order to obtain better detection results, it is often necessary to use several detection methods in combination
[0017] 1. The detection sensitivity is low, cytogenetics is 5%, FISH is 0.5%, and PCR method can reach 0.001-0.0001%, which is equivalent to 10000-100000 normal cells in one cell. -5 -10 -6 BCR-ABL fusion gene RNA expressed in individual cells
[0018] 2. The disadvantage of poor accuracy. The abnormal detection rate of traditional cytogenetic analysis is only 33.3%. When Ph+<10%, errors are prone to occur, and its sensitivity has been unable to detect minimal residual disease (MRD) in leukemia patients. testing, which limits the utility of cytogenetics in leukemia patients
[0020] 4. The detection cycle is long, the operation is complicated and the use of special instruments. The traditional cytogenetic method generally takes 1-2 weeks for chromosome culture only, and the cells must be cultured to the middle of cell division, which greatly increases the detection time. The culture technology requires high requirements, and the culture is sometimes prone to failure. Even if the culture is successful, some cells will not divide or the number of cell divisions will be insufficient, which will lead to the inability to detect positive results by conventional cytogenetics
The experimental operation of FISH detection is more complicated, it is difficult to detect when the specimen is not handled properly, and false positive results are prone to occur, the cost is high, and certain equipment is required, which makes it difficult to promote the clinical application of FISH
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0105] Embodiment 1 kit components and methods of use
[0106] Table 4 Nucleic Acid Amplification Kit 1
[0107]
[0108]
[0109] Table 5 Nucleic acid detection kit 1
[0110]
[0111]
[0112] Table 6 Nucleic acid detection kit 2
[0113]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present invention provides a BCR-ABL fusion gene amplification kit and a BCR-ABL fusion gene detection kit, wherein the amplification kit has advantages of convenient use, rapid operation, and high sensitivity. According to the present invention, the BCR-ABL fusion gene can be subjected to typing and quantitation with the detection kit, such that the pathogenetic condition development and the prognosis of the chronic myelocytic leukemia patient can be monitored according to the BCR-ABL fusion gene detection result.
Description
technical field [0001] The invention relates to the field of gene amplification, in particular to a fusion gene amplification kit and detection kit. Background technique [0002] Chronic myelogenous leukemia (CML) is a malignant clonal disease originating from hematopoietic stem cells. It is clinically divided into chronic phase, accelerated phase and blast phase. Acute phase is the main cause of clinical death. Cytogenetic characteristics are Ph Chromosome, namely t(9;22)(q34;q11), forms bcr-abl mRNA at the molecular level. t(9;22)(q34;q11) is a characteristic chromosomal translocation of CML, forming a breakpoint cluster-Abelson oncogene (bcr-abl) fusion gene, where bcr-abl p210 It is the molecular marker of CML. In recent years, foreign studies on the BCR-ABL fusion protein at the molecular level have found that it can be divided into three main types according to the position of the BCR breakpoint: M-bcr, m-bcr, μ-bcr, and the corresponding encoding p210 protein, p190 ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12Q1/68
CPCC12Q1/6886C12Q2600/156
Inventor 卿志荣郑帮
Owner SHANGHAI REPODX BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com