Application of dehydrogenated silibinin diether in preparation of medicaments for preventing and treating leukemia
A technology of dehydrogenated water and thripin ether, which is used in drug combinations, medical preparations containing active ingredients, anti-tumor drugs, etc. Small, simple method, huge social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
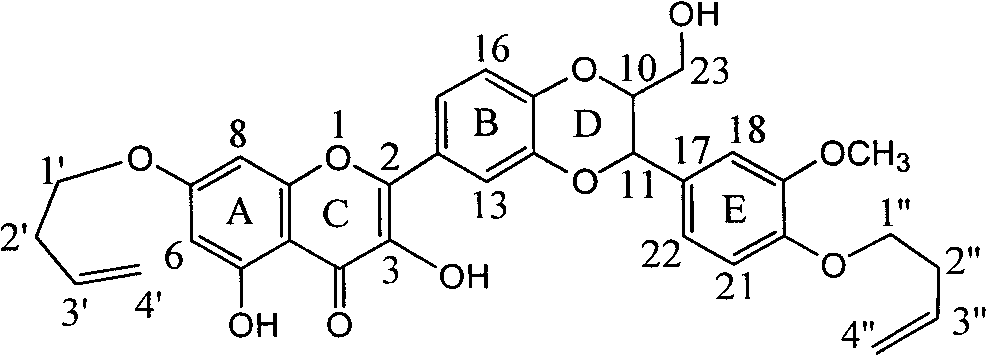
[0022] Example 1 : Preparation of 7,20-bis(1-butenyl)-dehydrosilibinin ether
[0023]
[0024] In a dry reaction flask, 0.241 g of silibinin was dissolved in 3 ml of DMF, 0.276 g of potassium carbonate was added, and stirred for 10 minutes to dissolve completely. 0.125 ml of 4-bromo-1-butene was slowly added dropwise, stirred for 10 minutes, and heated to reflux for 3 hours. Stand to cool, add 15 ml of distilled water, extract with ethyl acetate three times (20 ml each time), combine the organic layers, wash with 20 ml of distilled water, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The brownish-yellow crude product was obtained, and it was eluted with 200-300 mesh silica gel (20 g) with chloroform: ethyl acetate: acetic acid = 20:1:0.1 to obtain 68.8 mg of yellow crystals. Yield 23.7%.
[0025] 7,20-bis(1-butenyl)-dehydrosilibinin ether: 2-[2,3-dihydro-3-(4-enbutyloxy-3-methoxyphenyl) -2-Hydroxymethyl-1,4-benzodioxane-6-]-7-(enbutyloxy)-3,...
Embodiment 2
[0026] Example 2 : Inhibition of 7,20-bis(1-butenyl)-dehydrosilibinin on the proliferation of human chronic myelogenous leukemia cell line (K562) in vitro
[0027] 2.1 Cell culture:
[0028] Human chronic myelogenous leukemia (K562) cells were cultured with RPMI 1640 medium containing 10% calf serum, 100 U / ml penicillin and 100 U / ml streptomycin. cells in 5×10 per well 4 Seed into 96-well plates at 37 °C, 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0029] 2.2 Determination of cell viability using the modified MTT method:
[0030] After the cells were incubated for 24 hours, the dimethyl sulfoxide solution of the newly prepared compound 7,20-bis(1-butenyl)-dehydrosilibinin ether was added to each well in a concentration gradient, so that The final concentrations of the compound in the wells were 100 μg / ml, 50 μg / ml, 25 μg / ml, 5 μg / ml, respectively. After 72 hours, add 10 μl of MTT (5 mg / ml) in saline solution, and continue to incubate at 37°C, 5% CO 2 Cul...
Embodiment 3
[0034] Example 3 : Inhibition of 7,20-bis(1-butenyl)-dehydrosilibinin on the proliferation of human chronic myelogenous leukemia cell line doxorubicin-resistant (K562 / ADR) in vitro
[0035] 3.1 Establishment of human chronic myelogenous leukemia doxorubicin-resistant strain (K562 / ADR) cell line
[0036] Human chronic myelogenous leukemia (K562) cells were cultured with RPMI 1640 medium, containing 5% calf serum in the medium, adding 0.1 mg / L doxorubicin, and then gradually increasing the concentration of doxorubicin every two weeks until The final concentration of doxorubicin was 1.0 mg / L. Start the experiment after observing the stability of the cells. cells in 5×10 per well 4 Seed into 96-well plates at 37 °C, 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0037] 3.2 Use the modified MTT method to determine the growth inhibitory effect of positive drugs cisplatin and doxorubicin on K562 / ADR cells:
[0038] After the cells were incubated for 24 hours, the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com