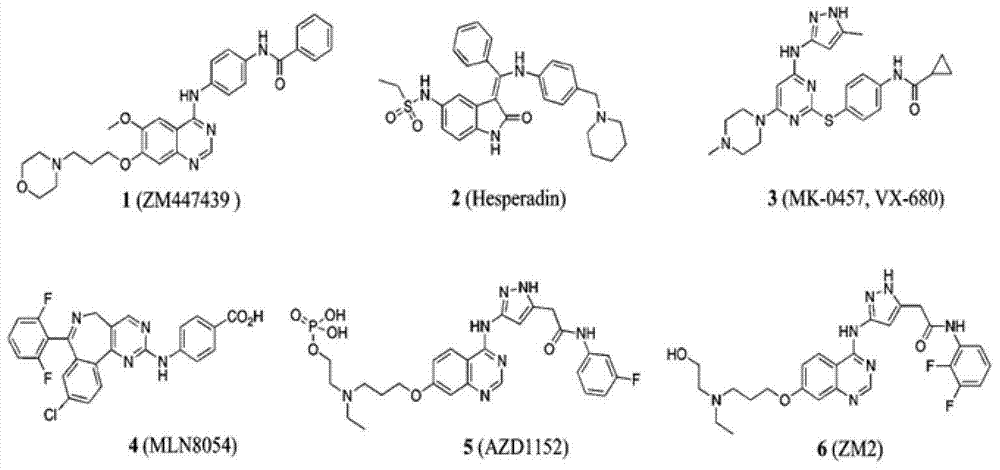
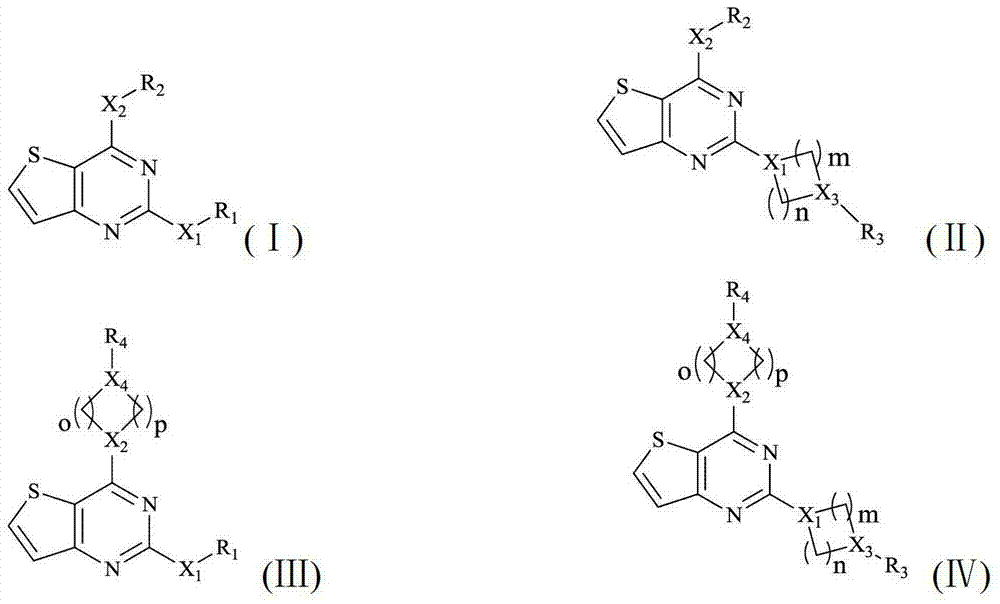
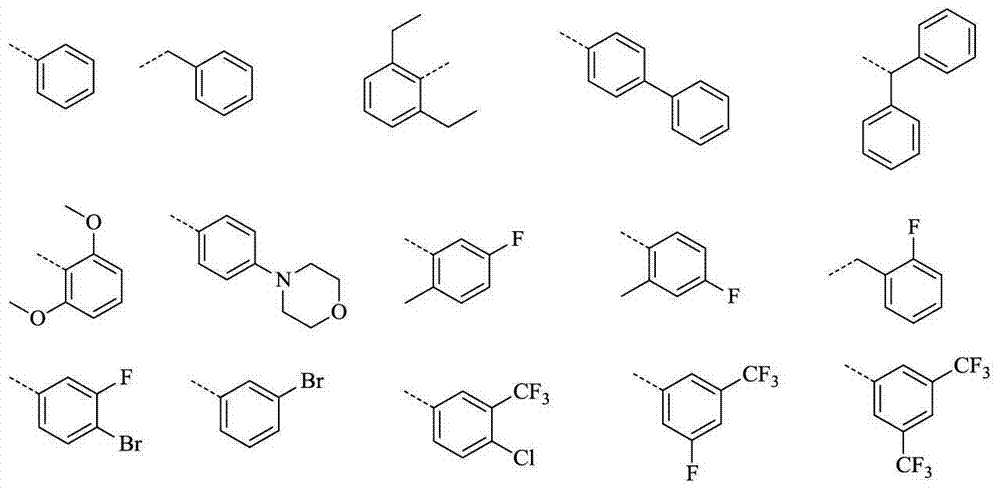
Thieno 2,4-substituted pyrimidine compound, and pharmaceutical composition and application thereof
A technology of compounds and pyrimidines, applied in drug combination, antipyretics, organic chemistry, etc., can solve the problems of drug-induced drug resistance gene mutation and narrow clinical application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1 N 4 -(5-Methyl-1H-pyrazole-3-substituted)-N 2 - Preparation of (3-methylbenzyl)thieno[3,2-d]pyrimidine-2,4-diamine (LD4484)
[0128] N 4 -(5-Methyl-1H-pyrazole-3-substituted)-N 2 -(3-methylbenzyl)thieno[3,2-d]pyrimidine-2,4-diamine The synthetic route is as follows:
[0129]
[0130]
[0131] Specifically include the following steps:
[0132] (1), the synthesis of thieno[3,2-d]pyrimidine-2,4-dione, its chemical structural formula is: The synthesis steps are:
[0133] Add methyl 3-aminopyrimidine-2-carboxylate (50 g, 0.318 mol) and urea (110 g, 1.96 mol) into a round bottom flask, heat to 150° C. and reflux for 8 hours. Cool down to 90°C, add water and stir overnight, filter and dry in vacuo to obtain a white solid, which is thieno[3,2-d]pyrimidine-2,4-dione (50.2 g, yield: 94%).
[0134] The characterization data of this compound are:
[0135] 1 H NMR (400MHz, d-DMSO), δ 11.22(t, J=8Hz, 2H), 8.04(m, J=6.8Hz, 1H), 6.90(m, J=6.4Hz, 1H).
[0136]...
Embodiment 2
[0151] Example 2 N 2 -(3-Bromobenzyl)-N 4 - Preparation of (5-methyl-1H-pyrazole-3-substituted)thieno[3,2-d]pyrimidine-2,4-diamines (LD4481)
[0152] N 2 -(3-Bromobenzyl)-N 4 The chemical structural formula of -(5-methyl-1H-pyrazole-3-substituted)thieno[3,2-d]pyrimidine-2,4-diamine is: Its synthesis steps are the same as in Example 1.
[0153] The characterization data of this compound are:
[0154] h 1 NMR (400MHz, d-DMSO), δ12.26(br, 1H), 10.31(br, 1H), 8.06(s, 1H), 7.98(br, 1H), 7.56(s, 1H), 7.42(d, J=7.6Hz, 1H), 7.35(t, J=14Hz, 1H), 7.28(t, J=15.2Hz, 1H), 7.16(d, J=5.2Hz, 1H), 6.21(br, 1H), 4.58(t, J=6Hz, 2H), 2.20(s, 3H).
[0155] MS (ESI), m / z: 416 (M + +H + ).
Embodiment 3
[0156] Example 3 N 2 -(4-Bromo-2-fluorobenzyl)-N 4 - Preparation of (5-methyl-1H-pyrazole-3-substituted)thieno[3,2-d]pyrimidine-2,4-diamines (LD4478)
[0157] N 2 -(4-Bromo-2-fluorobenzyl)-N 4 The chemical structure of -(5-methyl-1H-pyrazole-3-substituted)thieno[3,2-d]pyrimidine-2,4-diamine is Its synthesis steps are the same as in Example 1.
[0158] The characterization data of this compound are:
[0159] 1 H NMR (400MHz, d-DMSO), δ12.12(br, 1H), 9.69(br, 1H), 7.91(d, J=3.6Hz, 1H), 7.69(t, J=10.4Hz, 2H), 7.43(m, J=71.6Hz, 1H), 7.186(brs, 1H), 7.05(d, J=3.6Hz, 1H), 4.52(d, J=6Hz, 2H), 2.17(s, 3H).
[0160] MS (ESI), m / z: 434 (M + +H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com