Application of isorhamnetin in treatment of influenza
A technology of isorhamnetin and aspect, applied in the application field of isorhamnetin in the treatment of influenza, can solve problems such as unreported, and achieve the effect of improving inflammation and deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Isorhamnetin inhibits the replication of influenza virus
[0033] 1. Experimental materials
[0034] Cells: dog kidney cells (Madin Darby Canine Kidney, MDCK) cells
[0035] Virus: Influenza A PR8 strain (A / PR / 8 / 34, H1N1)
[0036] Reagents and drugs: fetal bovine serum (GIBCO, USA); MEM medium (GIBCO, USA); PBS (GIBCO, USA); TPCK trypsin (Sigma, USA); MTT kit (Sigma, USA); Sigma Company); Positive control drug oseltamivir, purchased from Bi De Pharmaceutical Company; isorhamnetin.
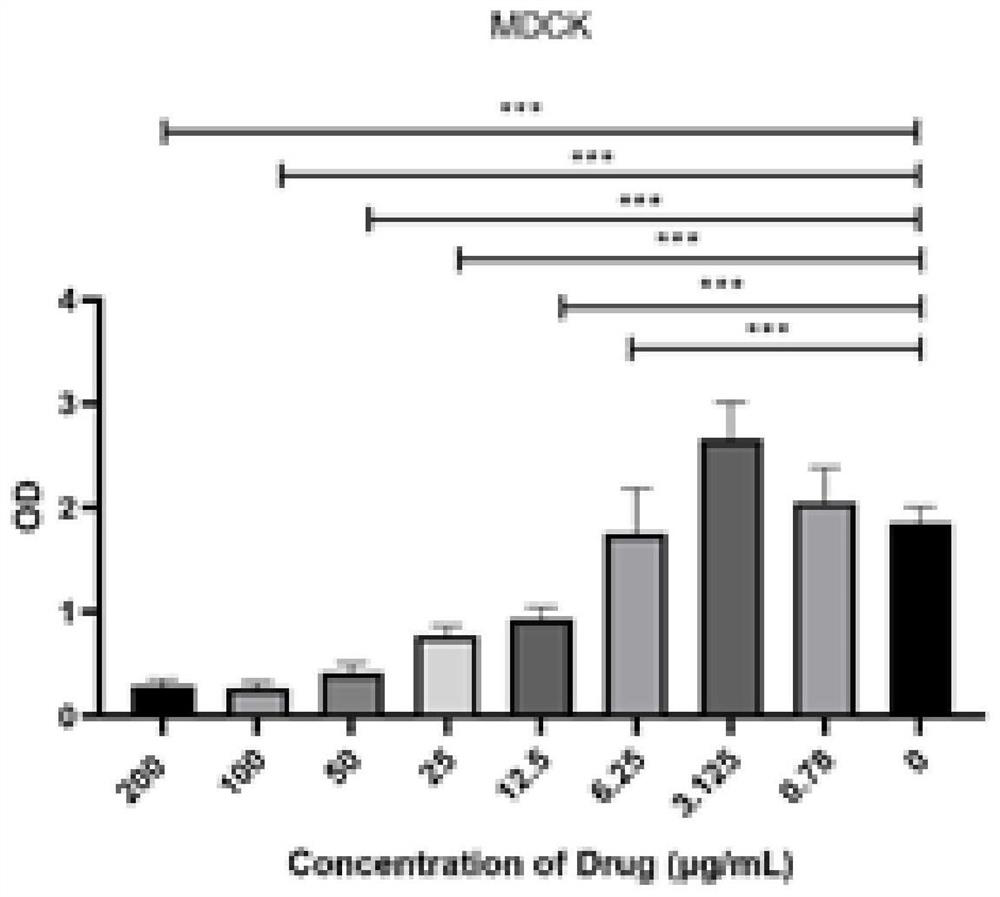
[0037] 2. Drug toxicity test (MIT method)
[0038] MDCK cells in the logarithmic growth phase were inoculated into 96-well plates, about 2.5×10 per well 4 After 24 hours, when the cells grow into a single layer, discard the culture medium, add 100 μL / well of drugs at different dilutions, add 100 μL / well of MEM to the blank control and normal cell control wells, and store at 37°C and 5% CO 2 After continuing to cultivate for 36 to 48 hours, add 20 μL of MTT solution (5 mg / mL) t...
Embodiment 2
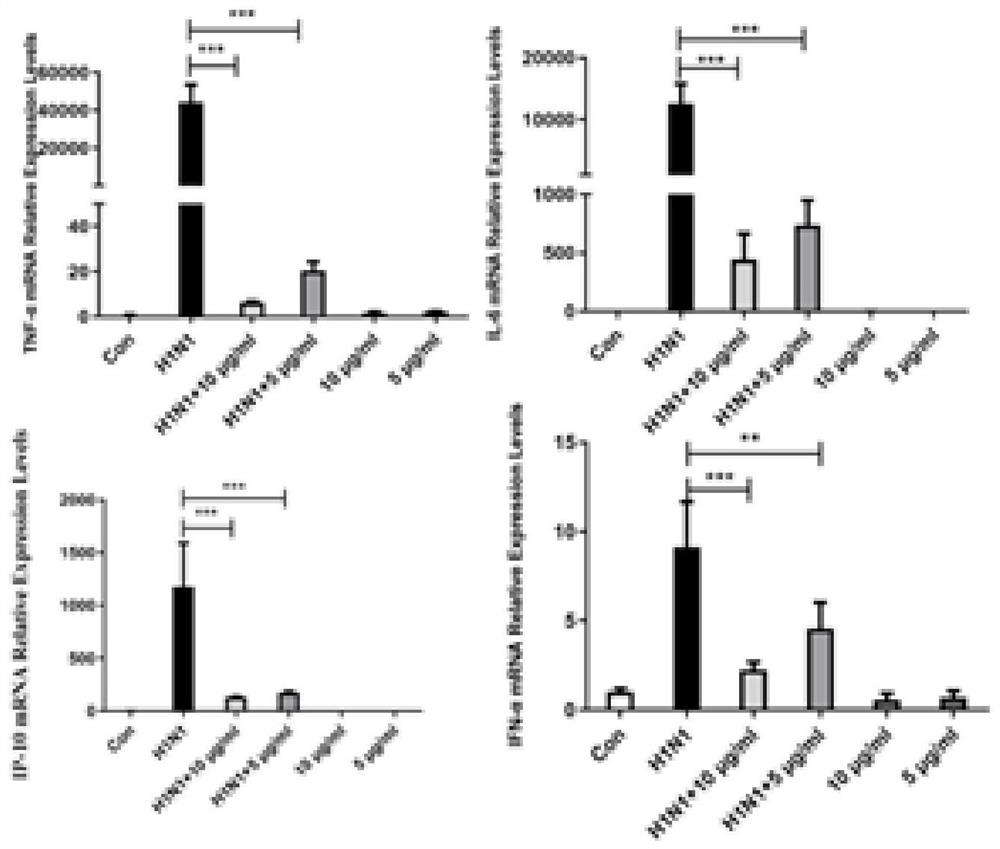
[0050] Example 2 Isorhamnetin inhibits the inflammatory response caused by influenza virus
[0051] 1. Experimental materials
[0052] Drug: Isorhamnetin.
[0053] Cells and viruses: human lung adenocarcinoma 549 cell line (A549), influenza A H1N1 virus (A / PR / 8 / 34, H1N1)
[0054] Reagents: DMEM medium; PBS and fetal bovine serum were purchased from GIBCO Company; TPCK was purchased from Guangzhou Jabbes Company; Reat-time PCR reagent was purchased from TaKarRa Company, Cat#RR390A; RIPA lysate was purchased from Sigma Company in the United States.
[0055] instrument:
[0056] HERAcell 150i constant temperature CO2 incubator, American Thermo Company;
[0057] BHC-1300IIA / B3 secondary biological safety cabinet, Suzhou Purification Equipment Co., Ltd.;
[0058] Leica DM3000 inverted microscope, Leica Corporation;
[0059] TGL-16M high-speed desktop refrigerated centrifuge, Eppendorf, Germany;
[0060] Avanti J-26 high-speed refrigerated centrifuge, BECKMAN COULTER, USA;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com