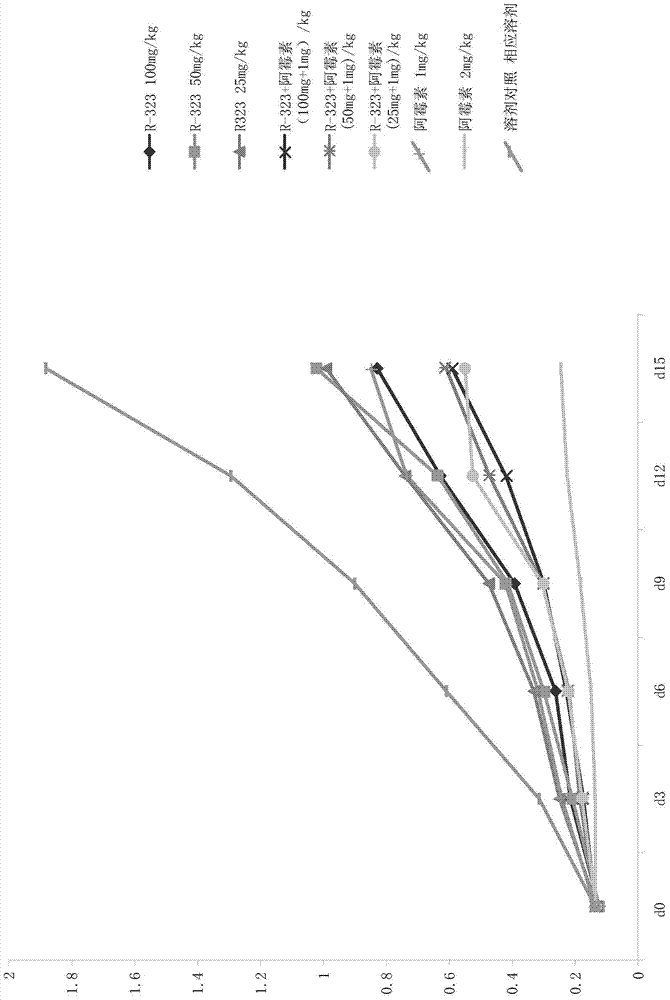
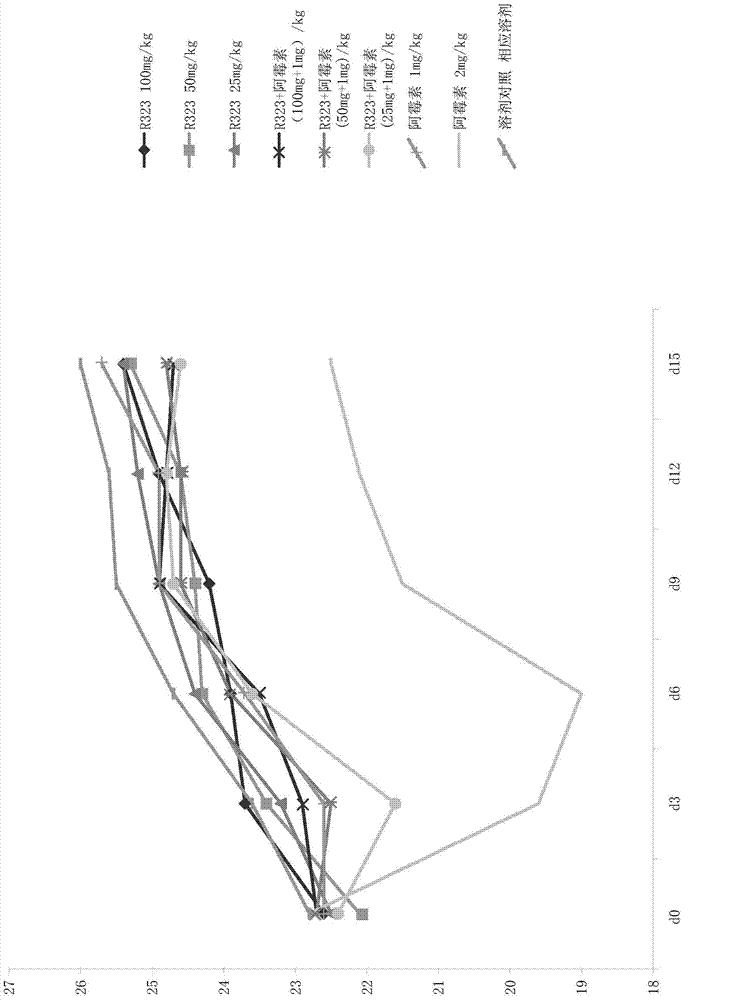
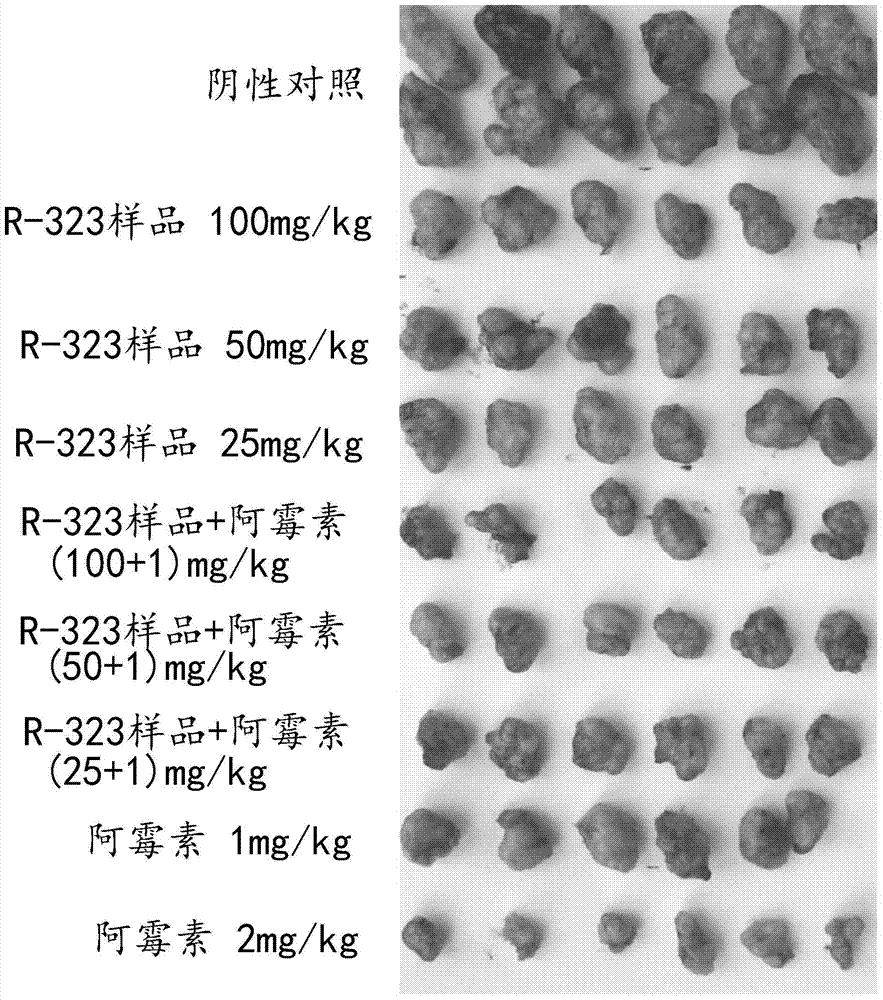
Use of dihydroartemisinin in preparation of drug for inhibition of tumour growth
A technology of dihydroartemisinin and tumor, applied in the field of anti-tumor drugs, can solve the problems of lack of iron ion anti-tumor effect, aggravate cardiotoxicity, etc., and achieve the effects of obvious anti-cancer effect, less side effects of chemotherapy and safe medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0230] Embodiment 1: Preparation of dihydroartemisinin tablet:
[0231] 1) Composition and dosage of raw and auxiliary materials:
[0232] Dihydroartemisinin 20g
[0233] Starch 55g
[0234] Lactose 55g
[0235] Sodium carboxymethyl starch 10g
[0236] Sodium Lauryl Sulfate 5g
[0237] Proper amount of povidone K30
[0238] Magnesium stearate 0.75g
[0239] Among them, dihydroartemisinin is a ready-made raw material purchased from a domestic pharmaceutical factory with GMP production qualification, and the quality standard of the raw material meets the international pharmacopoeia standard.
[0240] 2) Preparation process:
[0241] Dihydroartemisinin is passed through a 120 mesh sieve, and other auxiliary materials are passed through a 80 mesh sieve; Dihydroartemisinin is weighed according to the prescription amount, and starch, lactose, sodium carboxymethyl starch and sodium lauryl sulfate are mixed evenly; The 50% ethanol solution of % povidone K30 is a soft material ...
Embodiment 2
[0242] Embodiment 2: Preparation of dihydroartemisinin capsules:
[0243] 1) Composition and dosage of raw and auxiliary materials:
[0244] Dihydroartemisinin 20g
[0245] Starch 55g
[0246] Lactose 55g
[0247] Sodium carboxymethyl starch 10g
[0248] Sodium Lauryl Sulfate 5g
[0249] Proper amount of povidone K30
[0250] Magnesium stearate 0.75g
[0251] Among them, dihydroartemisinin is a ready-made raw material purchased from a domestic pharmaceutical factory with GMP production qualification, and the quality standard of the raw material meets the international pharmacopoeia standard.
[0252] 2) Preparation process:
[0253] Dihydroartemisinin is passed through a 120 mesh sieve, and other auxiliary materials are passed through a 80 mesh sieve; Dihydroartemisinin is weighed according to the prescription amount, and starch, lactose, sodium carboxymethyl starch and sodium lauryl sulfate are mixed evenly; The 50% ethanol solution of %povidone K30 is a soft material...
Embodiment 3
[0254] Embodiment 3: blank granule plus medicine mixing process:
[0255] Due to the poor thermal stability of dihydroartemisinin, during the preparation process, the heat generated by the frictional heat generation between the particles will also have an impact, so the present invention more preferably adopts the blank granule plus drug mixing process.
[0256] 1) Composition and dosage of raw and auxiliary materials:
[0257] Dihydroartemisinin 20g
[0258] Lactose 60g
[0259] Microcrystalline cellulose 60g
[0260] Sodium carboxymethyl starch 15g
[0261] Sodium Lauryl Sulfate 5g
[0262] Proper amount of povidone K30
[0263] Magnesium Stearate 0.5%
[0264] 2) Preparation process:
[0265] Dihydroartemisinin was pulverized at a low temperature below 20°C and passed through a 120-mesh sieve, and other excipients were passed through a 80-mesh sieve; lactose, microcrystalline cellulose, sodium carboxymethyl starch and sodium lauryl sulfate were weighed according to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com