Novel hydroxyl dihydroartemisinin derivative and application thereof
A technology for dihydroartemisinin and dihydroartemisinin ester, which is applied in the field of chemical medicine, can solve the problems of low bioavailability and poor solubility of artemisinin, and achieves simple preparation method, obvious anti-tumor activity, and obvious immunity. Inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
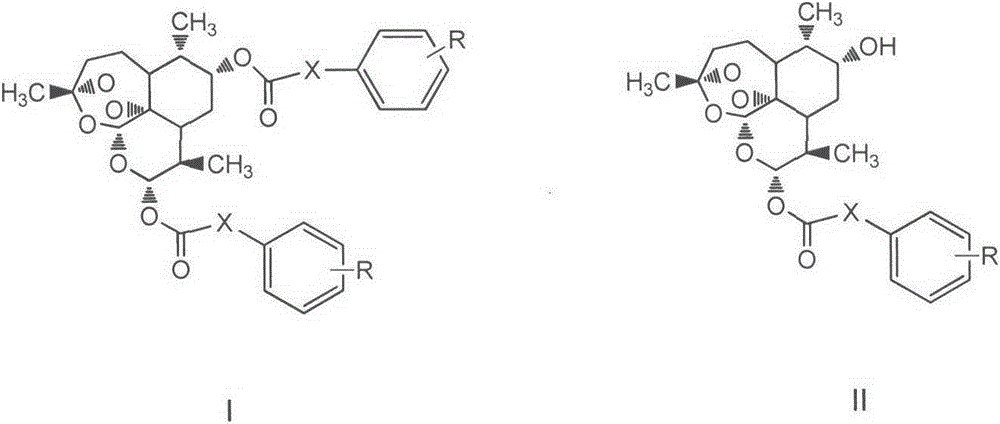
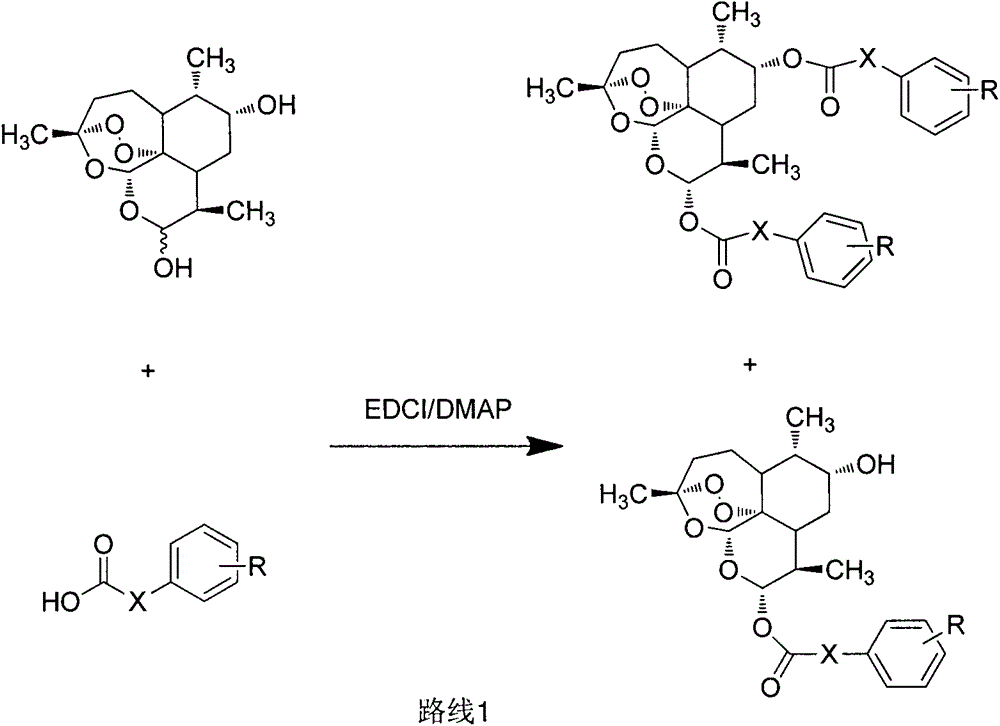
[0062] Example 1: Preparation of 9α, 12α-di-(cinnamic acid) dihydroartemisinin ester
[0063] Dissolve 150mg (0.5mmol) of 9α-hydroxydihydroartemisinin and 192.6mg (1.3mmol) of cinnamic acid in 10mL of anhydrous dichloromethane. Under nitrogen protection and ice bath conditions, 159mg (1.3mmol) of DMAP and 288mg (1.5mmol) EDCI was added in a three-neck round bottom flask, the temperature was natural, and the reaction was stirred for 12h. After the reaction was detected by TLC, it was spin-dried, dissolved in ethyl acetate, washed with water, washed with saturated sodium chloride, dried over anhydrous magnesium sulfate, and spin-dried. , the residue was subjected to column chromatography to obtain a white solid. Yield: 32%.
[0064] 1 HNMR (400MHz, Chloroform-d) δ = 7.79 (d, J = 16.0, 1H), 7.71 (d, J = 16.0, 1H), 7.57-7.53 (m, 4H), 7.42-7.38 (m, 6H), 6.49 (d, J=16.0, 1H), 6.44 (d, J=16.0, 1H), 5.94 (d, J=9.8, 1H), 5.60y (s, 1H), 4.64 (td, J=10.9, 4.4 , 1H), 2.69(ddd, J=9.9, ...
Embodiment 2
[0065] Example 2: Preparation of 9α, 12α-di-(p-methylcinnamic acid) dihydroartemisinin ester
[0066] According to the method of Example 1, the title compound was prepared by replacing the raw material cinnamic acid in Example 1 with p-methylcinnamic acid.
[0067] White powder, yield: 54%. 1 HNMR (400MHz, Chloroform-d) δ = 7.79 (d, J = 16.0, 1H), 7.71 (d, J = 16.0, 1H), 7.57-7.53 (m, 4H), 7.42-7.38 (m, 6H), 6.49 (d, J=16.0, 1H), 6.44 (d, J=16.0, 1H), 5.94 (d, J=9.8, 1H), 5.60y (s, 1H), 4.64 (td, J=10.9, 4.4 , 1H), 2.69(ddd, J=9.9, 7.1, 4.7, 1H), 2.49-2.37(m, 1H), 2.20-2.06(m, 2H), 1.93(dt, J=9.1, 4.4, 2H), 1.64-1.54(m, 4H), 1.46(d, J=3.8, 3H), 1.02(d, J=6.2, 3H), 0.91(d, J=7.1, 3H). 13 CNMR(101MHz,Chloroform-d)δ=166.51,165.42,146.25,145.32,134.28,130.53,130.46,128.94,128.23,128.15,117.87,117.46,104.60,91.80,91.22,79.15,75.46,49.08,42.77,41.39, 36.09, 31.71, 27.58, 25.92, 24.58, 15.44, 12.04. HRESIMS (m / z): 611.2615 [M+Na] + (calcdforC 35 h 40 NaO 8 , 611.2615).
Embodiment 3
[0068] Example 3: Preparation of 9α, 12α-di-(p-fluorocinnamic acid) dihydroartemisinin ester
[0069] According to the method of Example 1, the title compound was prepared by replacing the raw material cinnamic acid in Example 1 with p-fluorocinnamic acid.
[0070] White powder, yield 60%. 1 HNMR (400MHz, Chloroform-d) δ = 7.74 (d, J = 16.0, 1H), 7.66 (d, J = 16.0, 1H), 7.59-7.44 (m, 4H), 7.09 (td, J = 8.6, 4.2 , 4H), 6.41(d, J=15.9, 1H), 6.36(d, J=15.9, 1H), 5.93(d, J=9.8, 1H), 5.59(s, 1H), 4.63(td, J= 10.8, 4.4, 1H), 2.77-2.61(m, 1H), 2.50-2.34(m, 1H), 2.19-2.05(m, 2H), 1.98-1.89(m, 2H), 1.71-1.51(m, 4H ), 1.46(s, 3H), 1.01(d, J=6.2, 3H), 0.90(d, J=7.1, 3H). 13 CNMR(101MHz,Chloroform-d)δ=165.37,165.30,162.82,162.77,144.91,144.00,130.53,130.51,130.16,130.08,129.98,117.61,117.19,116.22,116.00,104.61,91.82,91.21,79.12,75.51, 49.06, 42.75, 41.37, 36.08, 31.69, 27.56, 25.90, 24.57, 15.43, 12.03. HRESIMS (m / z): 619.2107 [M+Na] + (calcdforC 33 h 34 f 2 NaO 8 , 619.2114). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com