Preparation method for two novel compounds with antitumor activity extracted from isodon excisoides and application of two novel compounds
A technology for simulating aroma-deficient tea vegetables and anti-tumor activity, applied in the field of medicine, can solve problems such as significant anti-tumor effect, and achieve the effects of strong orientation, high product purity, and fast separation speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
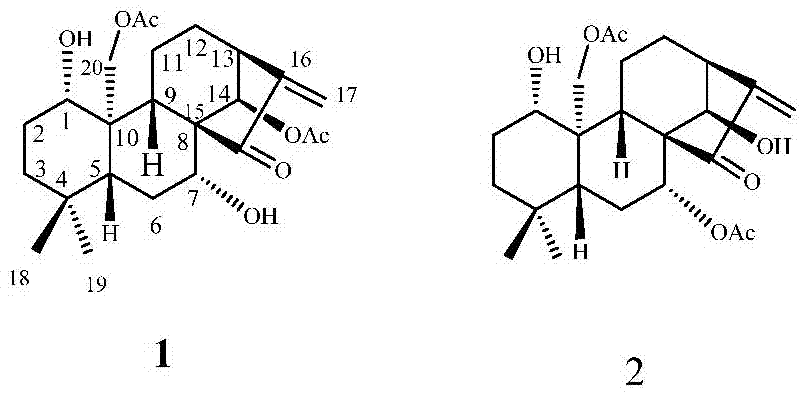
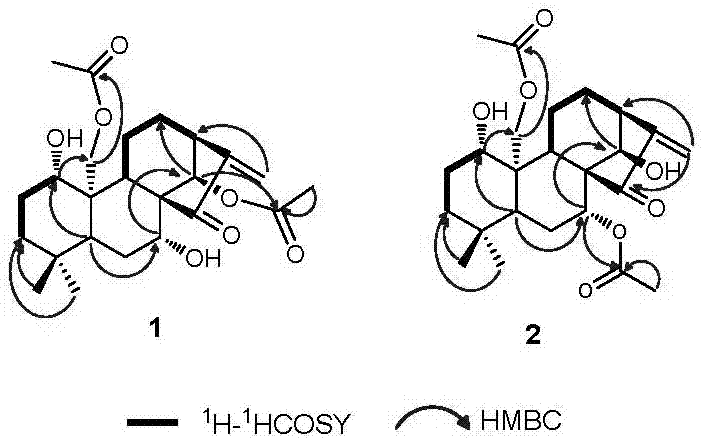
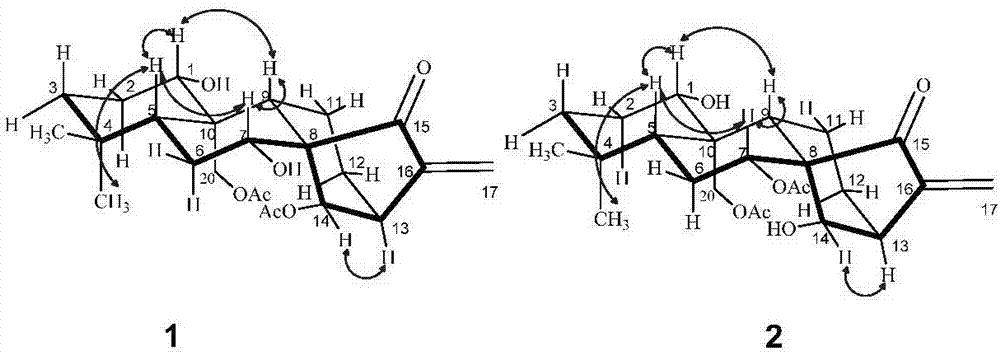
[0018] In the specific implementation of the present invention, compound 1 and compound 2 are two new compounds whose molecular formula is C 24 h 34 o 7 The degree of unsaturation is 8, and its molecular structure formula is figure 1 Given compound 1, the structural formula of compound 2, its preparation method is by Figure 4 shown, including the following steps:
[0019] (1) Dry and crush 9-11kg of the aboveground part of the tea-vegetable that lacks fragrance, add 280-350L of water each time, boil and extract 3 times at 100°C, extract 1.5h each time, combine the extracts 3 times, and concentrate to the equivalent of the crude drug 0.1g / mL concentrate;
[0020] (2) Load the concentrated solution on a D-101 macroporous adsorption resin column with a diameter-to-height ratio of 1:6, and perform gradient elution with 18L of water, 24L of 30% ethanol, 24L of 70% ethanol, and 12L of 95% ethanol in sequence , flow rate 3mL / min, get Fr.A, Fr.B, Fr.C, Fr.D four parts;
[0021]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com