Blood group A epitope mimic peptide and application thereof
An anti-tumor and DNA vaccine technology, applied in the field of biochemistry, can solve the problems of high cost, difficult to modify, and the properties of polysaccharide components are not stable enough.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Discovery and Screening of Example 1A Blood Group Antigen Epitope Mimetic Peptides
[0021] The A blood group antigen epitope mimetic peptide of the present invention is initially screened out from a phage random 12 peptide library, and the specific method is as follows:
[0022] 1. The titer determination of the phage random 12-peptide library ensures that the amount of phage input is about 1.5×10 11 pfu.
[0023] 2. Affinity panning method of phage random peptide library
[0024] (1) Coat 100 mg / L, 100 μl / well anti-blood group A antigen monoclonal antibody on the ELISA plate, shake gently in a wet box, and incubate overnight at 4°C. Take 10 μl of the peptide library stock solution and dilute it to 100 μl with TBS, add it to the wells of the ELISA plate, 100 μl / well, shake slowly at room temperature for 1 hour. Plates were washed 10 times with 0.1% TBST. Add 100 μl of 0.2M glycine-hydrochloric acid (pH2.2), dilute and amplify the eluate with LB medium, and measure ...
example 2
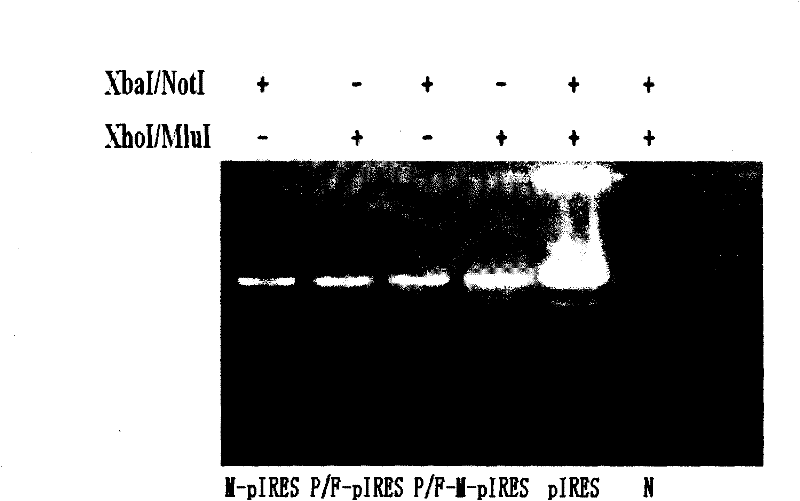
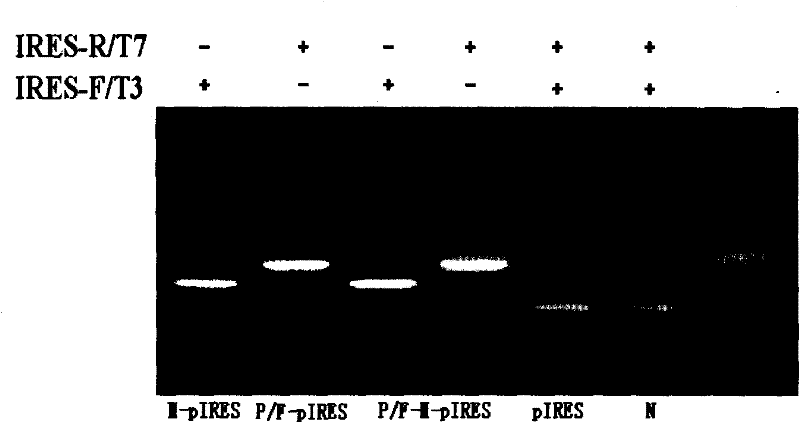
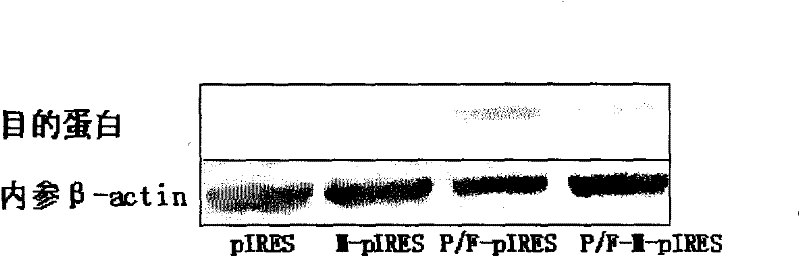
[0031] Example 2 Study on P / F-pIRES, P / F-M-pIRES Recombinant Plasmids and Their Antitumor Effects
[0032] 1. Construction of P / F-pIRES and P / F-M-pIRES recombinant plasmids
[0033] ①. Construction of P / F-pIRES recombinant plasmid
[0034] Using the sequence of SEQ NO.2 as a template, and using dATP, dGTP, dCTP and dTTP as raw materials, the transmembrane region of the mimetic peptide and FAS gene was formed in an ABI 394 DNA / RNA synthesizer through deprotection group activation, ligation, closure, oxidation and other steps And the P / F fusion gene fused with the intracellular segment, the upstream of the fusion gene contains the Xho I restriction site, and the downstream contains the Mlu I restriction site. The P / Fas fusion gene and pIRES plasmid were double digested with Xho I and Mlu I. The digestion reaction system was 40 μL, including: 8 μL 10×Tango Buffer, 2 μL Xho I, 2 μL Mlu I, 10 μL DNA and 18 μL sterile water. The reaction system was vortexed and mixed, then placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com