2-alkyl-benzimidazole-1-acetyl hydrazone and its application
A technology of benzimidazole and acetyl, which is applied in the field of benzimidazole compounds and their derivatives, can solve the problems that the synthesis and application of benzimidazole hydrazone compounds have not been reported in literature, and achieve high atom utilization and high yield , easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
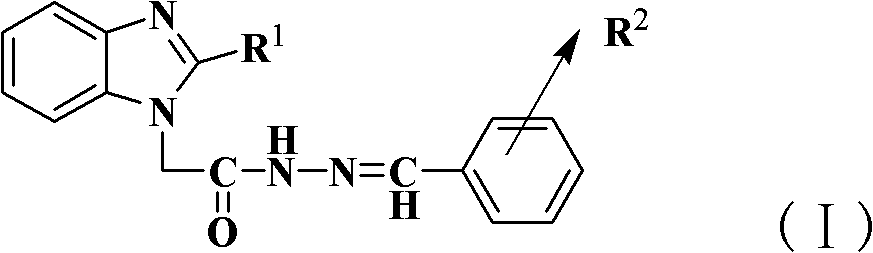
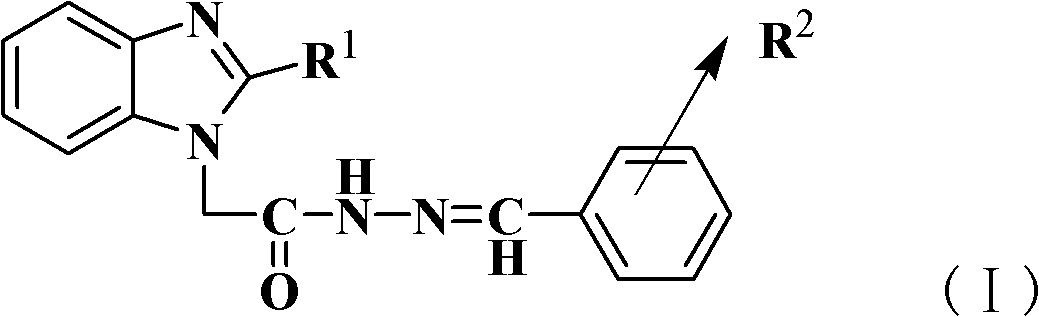
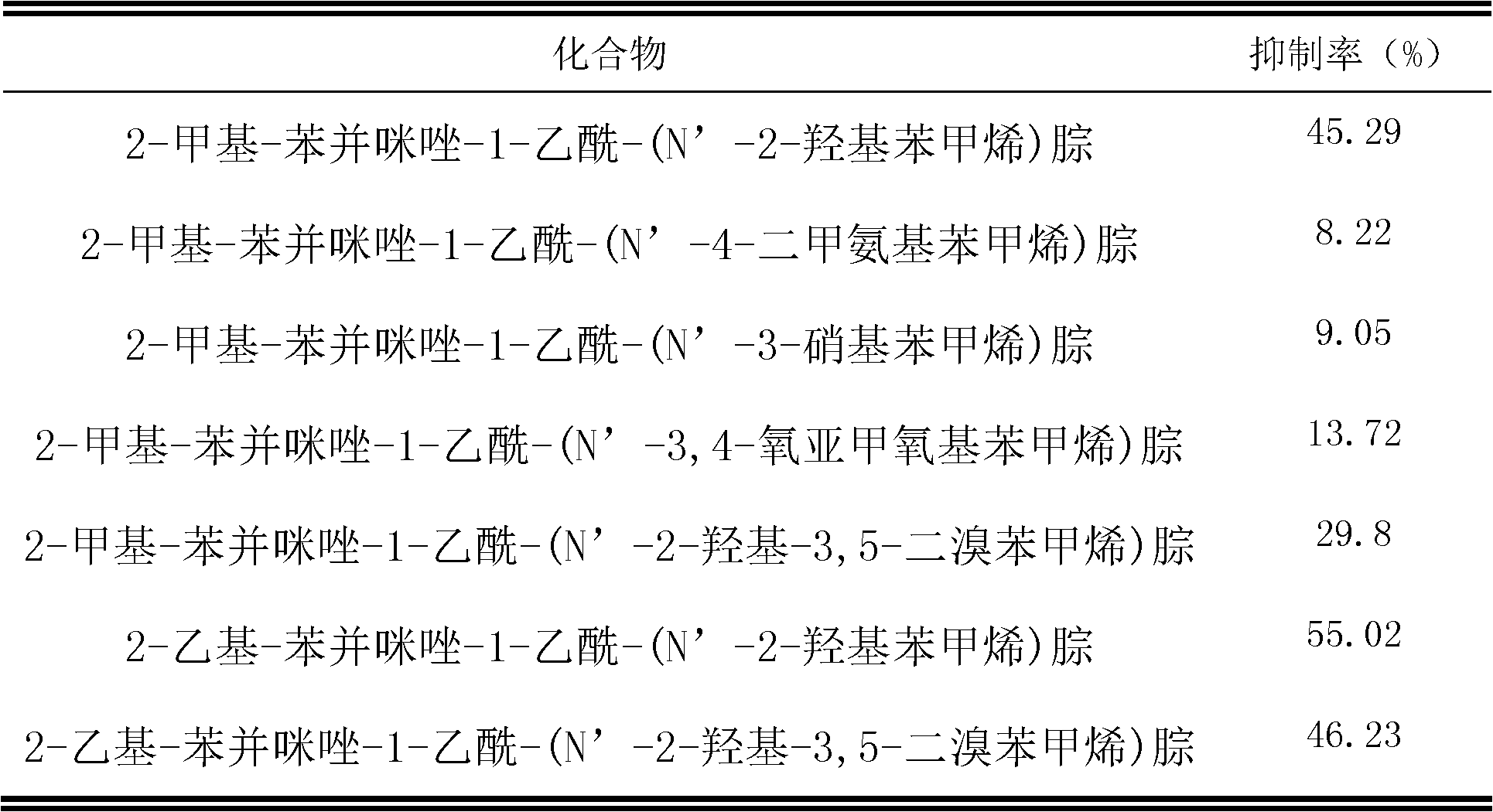
[0025] Add 2-methyl-benzimidazole-1-acetylhydrazide (1mmol, 0.21g) and ethanol solution (95%, 10g) to the reactor, heat and stir until the hydrazide is completely dissolved, then add salicylaldehyde (1mmol) , and finally add 0.35mmol p-toluenesulfonic acid, after adding all, heat to reflux for 5h. Finish the reaction, concentrate under reduced pressure to obtain 2-methyl-benzimidazole-1-acetyl-(N'-2-hydroxybenzyl)hydrazone, and then use ethanol-water with a volume ratio of ethanol and water of 1:4 Recrystallization gave white crystals; yield 75%; m.p.>300°C; IR(KBr): 3466(ν-O-H), 3208(ν-N-H), 1680(ν-C=O), 1614(ν-C =N), 1481 (Ar-ring), 1291 (ν-C-O); 1 H-NMR (DMSO): δ: 2.47(s, 3H), 5.03 / 5.46(s, 2H), 6.86-6.93(m, 2H), 7.13-7.18(m, 2H), 7.25-7.29(t, 1H ), 7.44-7.47(m, 1H), 7.52-7.57(m, 1H), 7.82-7.84(d, 1H), 8.38 / 8.49(s, 1H), 10.05 / 10.88(s, 1H), 1.70 / 12.07 (s, 1H); elemental analysis (C 17 h 16 N 4 o 2 ), calculated % (measured %): C: 66.21 (66.49), H: 5.24 (5.16), N: 18.17...
Embodiment 2
[0027] Add 2-methyl-benzimidazole-1-acetylhydrazide (1mmol, 0.21g) in the reactor, ethanol solution (95%, 10g), heat and stir until the hydrazide is completely dissolved, then add salicylaldehyde (1.25mmol ), and finally add 0.35mmol acetic acid, after adding all, heat to reflux for 16h. Finish the reaction, concentrate under reduced pressure to obtain 2-methyl-benzimidazole-1-acetyl-(N'-2-hydroxybenzyl)hydrazone, and then use ethanol-water with a volume ratio of ethanol and water of 1:4 Recrystallization gave white crystals; yield 36%; m.p.>300°C; IR (KBr): 3466 (ν-O-H), 3208 (ν-N-H), 1680 (ν-C=O), 1614 (ν-C =N), 1481 (Ar-ring), 1291 (ν-C-O); 1 H-NMR (DMSO): δ: 2.47(s, 3H), 5.03 / 5.46(s, 2H), 6.86-6.93(m, 2H), 7.13-7.18(m, 2H), 7.25-7.29(t, 1H ), 7.44-7.47(m, 1H), 7.52-7.57(m, 1H), 7.82-7.84(d, 1H), 8.38 / 8.49(s, 1H), 10.05 / 10.88(s, 1H), 1.70 / 12.07 (s, 1H); elemental analysis (C 17 h 16 N 4 o 2 ), calculated % (measured %): C: 66.21 (66.49), H: 5.24 (5.16), N: 18.17 (18....
Embodiment 3
[0029] Add 2-methyl-benzimidazole-1-acetylhydrazide (1mmol, 0.21g), ethanol (95%, 10g) successively in the reactor, heat and stir until the hydrazide is completely dissolved, then add salicylaldehyde (1.15mmol ), and finally added 0.35mmol HCl, after all the addition, heated to reflux for 9h. Finish the reaction, concentrate under reduced pressure to obtain 2-methyl-benzimidazole-1-acetyl-(N'-2-hydroxybenzyl)hydrazone, and then use ethanol-water with a volume ratio of ethanol and water of 1:4 Recrystallization gave white crystals; yield 52%; m.p.>300°C; IR (KBr): 3466 (ν-O-H), 3208 (ν-N-H), 1680 (ν-C=O), 1614 (ν-C =N), 1481 (Ar-ring), 1291 (ν-C-O);1 H-NMR (DMSO): δ: 2.47(s, 3H), 5.03 / 5.46(s, 2H), 6.86-6.93(m, 2H), 7.13-7.18(m, 2H), 7.25-7.29(t, 1H ), 7.44-7.47(m, 1H), 7.52-7.57(m, 1H), 7.82-7.84(d, 1H), 8.38 / 8.49(s, 1H), 10.05 / 10.88(s, 1H), 1.70 / 12.07 (s, 1H); elemental analysis (C 17 h 16 N 4 o 2 ), calculated % (measured %): C: 66.21 (66.49), H: 5.24 (5.16), N: 18.17 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com