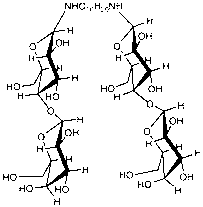
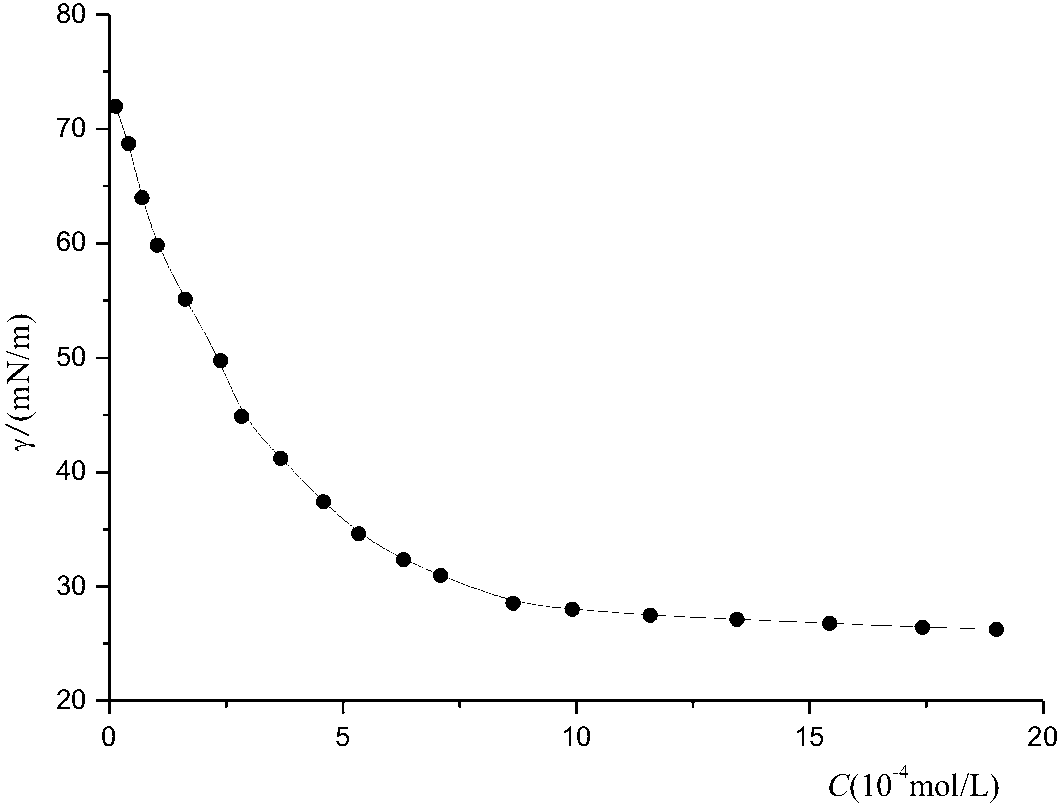
Method for directly preparing N,N-bislactose-1,10-decamethylene diamine by adopting one-pot reaction
A decanediamine, lactose-based technology, applied in the field of sugar-based surfactant synthesis, can solve the problems of difficult control of reaction conditions, complex reaction, harsh conditions, etc., and achieves high implementation value, simple post-processing, simple and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
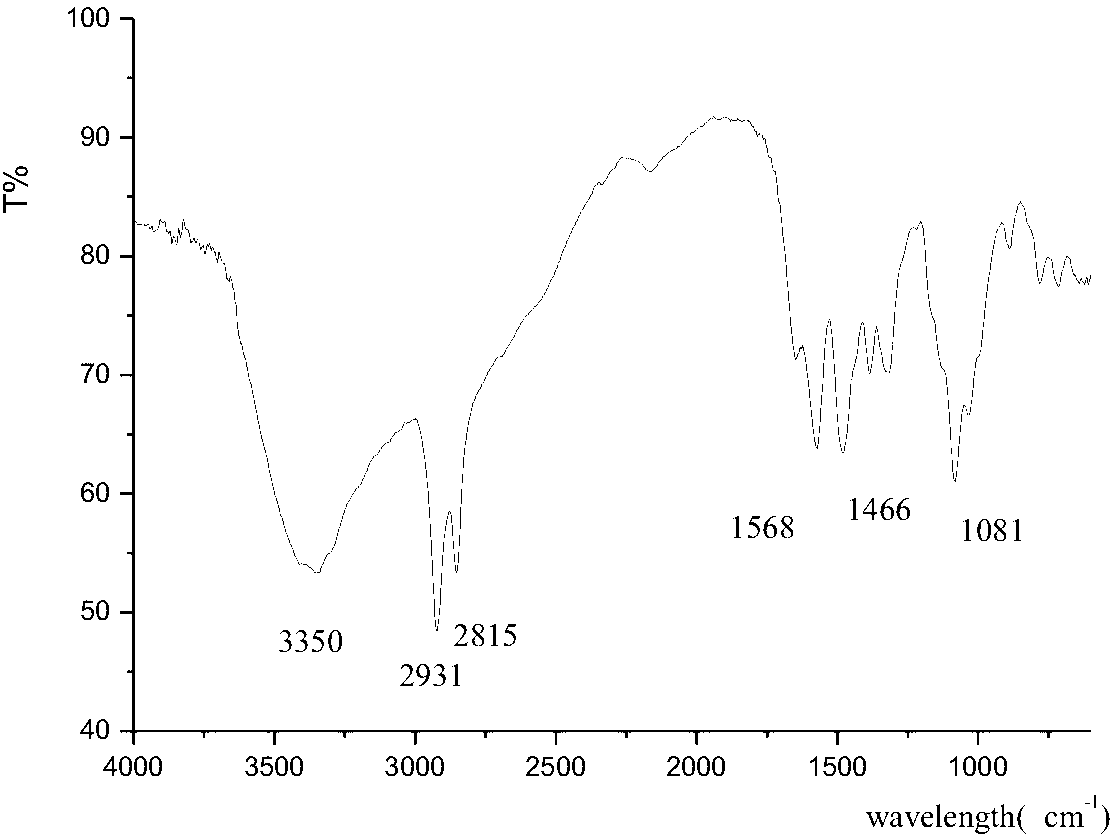
[0013] This example is the synthesis of N,N'-dilactosyl-1,10-decanediamine. In a 250mL three-necked flask equipped with a condenser, add 10.269g (30mmol) of lactose, 2.585g (15mmol) of decanediamine, and 51g of an alcohol-water mixed solvent with a volume ratio of methanol:water of 50:100. Stir mechanically at room temperature for 20 hours, then heat in a water bath to 45°C for 2 hours, cool and place for 0.5 hours, a large amount of solid precipitates, filter with suction, wash with ether, acetone, and ethanol in sequence to obtain a light yellow solid. Then recrystallized with absolute ethanol at 60° C., recrystallized three times, and freeze-dried to obtain a white solid with a mass of 7.389 g (9 mmol) and a yield of 60%. of the resulting product 1 H-NMR (DMSO): δ1.35 corresponds to the 16 protons on the 8 methylene (16H) in the long chain of the alkyl group, and δ1.45 corresponds to the 2 methylenes connected to the secondary amino group in the long chain of the alkyl gro...
Embodiment 2
[0015] This example is the synthesis of N,N'-dilactosyl-1,10-decanediamine. In a 500mL three-neck flask equipped with a condenser, add 56.480g (165mmol) of lactose, 12.924g (75mmol) of decanediamine, and 347g of an alcohol-water mixed solvent with a volume ratio of isopropanol:water of 67:100. Stir mechanically at room temperature for 22 hours, then heat in a water bath to 50°C for 2 hours, cool and place for 2 hours, a large amount of solid precipitates, filter with suction, wash with ether, acetone, and ethanol in sequence to obtain a light yellow solid. Then recrystallized with absolute ethanol at 60° C., recrystallized three times, and freeze-dried to obtain a white solid with a mass of 44.334 g (54 mmol) and a yield of 72%. Product structure characterization results are the same as in Example 1.
Embodiment 3
[0017] This example is the synthesis of N,N'-dilactosyl-1,10-decanediamine. In a 500mL three-necked flask equipped with a condenser, add 35.428g (103.5mmol) of lactose, 7.754g (45mmol) of decanediamine, and 259g of an alcohol-water mixed solvent with a volume ratio of ethanol:water of 75:100. Stir mechanically at room temperature for 24 hours, then heat in a water bath to 55°C for 1 hour, cool and place for 1 hour, a large amount of solid precipitates, filter with suction, wash with ether, acetone, and ethanol in sequence to obtain a light yellow solid. Then recrystallized with absolute ethanol at 60°C, recrystallized three times, and freeze-dried to obtain a white solid with a mass of 27.709 g (33.75 mmol) and a yield of 75%. Product structure characterization results are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com